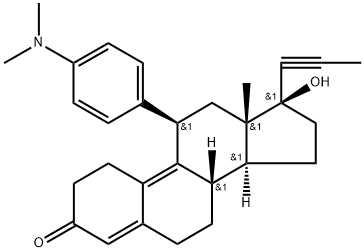
Мифепристон
- английское имяMifepristone
- CAS №84371-65-3
- CBNumberCB6351940
- ФормулаC29H35NO2
- мольный вес429.59
- EINECS617-559-7
- номер MDLMFCD00867226
- файл Mol84371-65-3.mol
химическое свойство
| Температура плавления | 195-198°C |
| альфа | D20 +138.5° (c = 0.5 in chloroform) |
| Температура кипения | 544.13°C (rough estimate) |
| плотность | 1.0731 (rough estimate) |
| показатель преломления | 1.6290 (estimate) |
| температура хранения | 2-8°C |
| растворимость | Soluble in DMSO (up to 40 mg/ml) or in Ethanol (up to 20 mg/ml). |
| форма | Yellow solid |
| пка | 12.94±0.60(Predicted) |
| цвет | Yellow |
| Биологические источники | synthetic (organic) |
| Растворимость в воде | 474.8ug/L(22.5 ºC) |
| Мерк | 14,6186 |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| ИнЧИКей | VKHAHZOOUSRJNA-GCNJZUOMSA-N |
| Справочник по базе данных CAS | 84371-65-3(CAS DataBase Reference) |
| FDA UNII | 320T6RNW1F |
| Словарь онкологических терминов NCI | Mifeprex; mifepristone; RU 486 |
| Словарь наркотиков NCI | Mifeprex |
| Код УВД | G03XB01,G03XB51 |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
больше
| Коды опасности | T |
| Заявления о рисках | 60-61 |
| Заявления о безопасности | 53-22-36/37/39-45 |
| WGK Германия | 3 |
| RTECS | KG2955000 |
| кода HS | 29372900 |
| Банк данных об опасных веществах | 84371-65-3(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H360:Может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Мифепристон химические свойства, назначение, производство
Описание
Mifepristone is an orally-active progesterone and glucocorticoid receptor antagonist indicated for use as a post-coital contraceptive. In addition to being an abortifacient, mifepristone is reported to be effective in the treatment of ocular hypertension; its potential therapeutic effect in hormone-dependent tumors is currently under investigation.Химические свойства
Pale Yellow SolidИспользование
Mifepristone is a progesterone receptor antagonist with partial agonist activity. Abortifacient.Показания
Mifepristone is a progesterone receptor antagonist that has a high affinity for glucocorticoid receptors and little agonist effect.This drug has recently been approved for use in the United States for the treatment of hypercortisolism. At high doses, mifepristone blocks negative feedback of the hypothalamic–pituitary axis, thereby increasing endogenous corticotrophin and cortisol levels. Because mifepristone exerts its effects at the receptor level and not by altering glucocorticoid production, elevated serum cortisol and corticotrophin levels may not accurately reflect the effectiveness of the therapeutic regimen. Mifepristone does not inhibit cortisol binding to the mineralocorticoid receptor, so that the resulting corticotrophin disinhibition may cause potassium depletion. Thus, administration of a mineralocorticoid receptor antagonist such as spironolactone may be indicated with mifepristone. Hypoadrenalism, nausea, and drowsiness have been reported during prolonged administration of mifepristone.Всемирная организация здравоохранения(ВОЗ)
Mifepristone, an antiprogesterone used in combination with a prostaglandin for the termination of early pregnancy, was introduced in 1990. Use of the combination has been associated with episodes of coronary spasm that are attributed to administration of the prostaglandin and which have resulted in several cases of cardiac infarction and ventricular fibrillation. At least one of these incidents has been fatal.Биологическая активность
Selective antagonist at progesterone (PR) and glucocorticoid (GR) receptors in vitro and in vivo . Is a silent antagonist at PR and has a higher affinity than progesterone. Has higher affinity for GR than dexamethasone.Фармакокине?тика
Following oral administration, mifepristone is rapidly absorbed, with a peak plasmaconcentration in approximately 90 minutes , an oral bioavailability of approximately 70%, and a term inal elimination half-life of 18 hours. It is 98% protein bound, primarily to album in and α1-acid glycoprotein. Mifepristone is metabolized primarily via CYP3A4 pathways involving mono- and di-N-demethylation and terminal hydroxylation of the 17-propynyl chain. The fact that approximately 83% of the drug is recovered in the feces and 9% in the urine suggests a biliary route of elimination. Mifepristone also demonstrates antiglucocorticoid activity.Клиническое использование
An antiprogestin is a substance that competes with progesterone for its receptor and, ultimately, prevents progesterone from binding to and activating its receptor. Because progesterone is integral to the continuation of an early pregnancy, it is expected that antipro-gestins will interfere with pregnancy maintenance. In 1982, the first antiproges tin, mifepristone (RU 486), was reported. Mifepristone was shown to interrupt early stages of implantation and pregnancy in humans.использованная литература
https://pubchem.ncbi.nlm.nih.gov/compound/mifepristone#section=Tophttps://www.drugbank.ca/drugs/DB00834
https://www.drugs.com/cdi/mifepristone-tablets.html
Мифепристон запасные части и сырье
Мифепристон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613315996897 | China | 792 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-18565342920; +8618565342920 |
China | 290 | 58 | ||
| +undefined17712220823 | China | 615 | 58 | ||
| +86-13082019107 +86-13082019107 |
China | 244 | 58 | ||
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 |