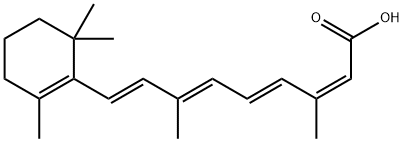
Изотретиноин
- английское имяIsotretinoin
- CAS №4759-48-2
- CBNumberCB6222631
- ФормулаC20H28O2
- мольный вес300.44
- EINECS225-296-0
- номер MDLMFCD00079542
- файл Mol4759-48-2.mol
| Температура плавления | 172-175 °C (lit.) |
| Температура кипения | 381.66°C (rough estimate) |
| плотность | 1.0597 (rough estimate) |
| показатель преломления | 1.4800 (estimate) |
| температура хранения | -20°C |
| растворимость | Practically insoluble in water, soluble in methylene chloride, slightly soluble in ethanol (96 per cent). It is sensitive to air, heat and light, especially in solution. Carry out all operations as rapidly as possible and avoid exposure to actinic light; use freshly prepared solutions. |
| пка | 4.76±0.33(Predicted) |
| форма | Fine Crystalline Powder |
| цвет | Yellow-orange to orange |
| Биологические источники | synthetic |
| Растворимость в воде | insoluble |
| λмакс | 354nm(EtOH)(lit.) |
| Мерк | 14,5228 |
| Стабильность | Stable, but probably air and light sensitive. Combustible. Incompatible with strong oxidizing agents. |
| ИнЧИКей | SHGAZHPCJJPHSC-YCNIQYBTSA-N |
| Справочник по базе данных CAS | 4759-48-2(CAS DataBase Reference) |
| Словарь онкологических терминов NCI | 13-cis retinoic acid; isotretinoin |
| FDA UNII | EH28UP18IF |
| Словарь наркотиков NCI | Accutane |
| Код УВД | D10AD04,D10AD54,D10BA01 |
| Предложение 65 Список | Isotretinoin |
| Система регистрации веществ EPA | Isotretinoin (4759-48-2) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | T | |||||||||
| Заявления о рисках | 61-36/37/38-20/21/22 | |||||||||
| Заявления о безопасности | 53-26-36/37/39-45-37/39 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | VH6440000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29362100 | |||||||||
| Банк данных об опасных веществах | 4759-48-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 (20 day) in mice, rats (mg/kg): 904, 901 i.p.; 3389, >4000 orally (Kamm) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H360:Может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Изотретиноин химические свойства, назначение, производство
Описание
Isotretinoin is the 9-cis isomer of retinoic acid, a close relative of retinol, or vitamin A. It was originally developed to treat cystic acne, and today this is still its primary use despite several more modern applications of the drug, including a treatment for pancreatic and brain cancers. First shown to be an effective treatment for acne in 1982, its development stemmed from advances in knowledge of the effects of vitamin A to reduce or eliminate sebum production. Since that time, however, several instances of deleterious effects became well known, most notably birth defects arising from the use of isotretinoin.Химические свойства
Yellow or orange crystalline powder or crystal, insoluble in water, slightly soluble in ethanol, very slightly soluble in ether, soluble in chloroform.Использование
isotretinoin is a retinoid derivative with improved bioavailability and percutaneous absorption for acne treatment products. Presently being studied in conjuction with the treatment of photoaged skin.Подготовка
Preparation of isotretinoin in a single step from β-ionone and ethyl chloride are first reacted together after which the product is further reacted with triphenylphosphine to obtain Triphenyl salt. The Triphenyl salt is further reacted with cyclopentenone derivative to produce isotretinoin and its 8Z isomer. separate out the 8Z isomer and convert it to isotretinoin through isomerization with the help of nitric acid.Показания
Isotretinoin (Accutane) alters keratinization in the acroinfundibulum of sebaceous glands and shrinks them, thereby reducing sebum excretion and comedogenesis. These features underlie its usefulness in acne vulgaris, since sebum secretion is a hallmark of acneprone skin. Furthermore, the drug has antiinflammatory activity.Определение
ChEBI: Isotretinoin is a retinoic acid that is all-trans-retinoic acid in which the double bond which is alpha,beta- to the carboxy group is isomerised to Z configuration. A synthetic retinoid, it is used for the treatment of severe cases of acne and other skin diseases. It has a role as a keratolytic drug, an antineoplastic agent and a teratogenic agent. It is a conjugate acid of a 13-cis-retinoate.Всемирная организация здравоохранения(ВОЗ)
Isotretinoin, a retinol derivative, was introduced in 1982 exclusively for the treatment of severe acne. Its use in pregnant women has resulted in major fetal abnormalities. The manufacturer's information emphasizes that the drug is teratogenic and must not be given to women who are pregnant, and that contraceptive measures must be maintained for at least four weeks after discontinuation of treatment. In some countries, blood banks are advised not to accept as donors persons who have taken isotretinoin within the previous four weeks. See also under retinol (vitamin A).Общее описание
Yellow-orange to orange crystalline powder; orange-brown chunky solid.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
An organic acid and unsaturated aliphatic hydrocarbon. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.Пожароопасность
Flash point data for Isotretinoin are not available; however, Isotretinoin is probably combustible.Механизм действия
Isotretinoin binds to and activates nuclear retinoic acid receptors (RARs); activated RARs serve as transcription factors that promote cell differentiation and apoptosis. This agent also exhibits immunomodulatory and anti-inflammatory responses and inhibits ornithine decarboxylase, thereby decreasing polyamine synthesis and keratinization. Isotretinoin is rapidly absorbed orally, with peak blood concentrations 3 hours after ingestion. It is not stored in tissue, and the elimination half-life is 10 to 20 hours, either after a single dose or during chronic therapy.Клиническое использование
Isotretinoin is most useful for the treatment of severe recalcitrant nodular acne vulgaris. It may also be helpful in other disorders of keratinization, but it is not useful for psoriasis. High doses of isotretinoin (2mg/kg/day) are effective as cancer chemoprevention agents to reduce the frequency of cutaneous malignancies in patients at increased risk, such as those with xeroderma pigmentosum, an inherited disorder in which DNA repair is deficient, or in immunosuppressed patients.Побочные эффекты
Isotretinoin is teratogenic to humans and should not be administered to pregnant women or women contemplating pregnancy. Concomitant use of isotretinoin with drugs of the tetracycline class increases the incidence of Pseudotumor cerebri. There have been recent reports of an increased risk of depression, suicide, and suicide attempts in individuals taking isotretinoin, but the causality has not been absolutely proved.Isotretinoin, like many retinoids, can lead to increase in serum aminotransferase levels, but, unlike acitretin and etretinate, isotretinoin has not been clearly implicated in cases of clinically apparent acute liver injury with jaundice.
Профиль безопасности
Poison by intraperitoneal route. Moderately toxic by ingestion. A human teratogen by ingestion with fetal developmental abnormalities of the skin and appendages and other postnatal effects. Human reproductive effects. Human systemic effects: decreased immune response, darrhea, hypermouhty, irritative dermatitis, sweating. Human mutation data reported. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.Изотретиноин запасные части и сырье
Изотретиноин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 0917-3909592 13892490616 |
China | 9310 | 58 | ||
| +8613073028829 | China | 2994 | 58 | ||
| +8617762501948 | China | 153 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +8618873369619 | China | 260 | 58 | ||
| +86-15373193816 +86-15373193816 |
China | 269 | 58 | ||
| +8613343047651 | China | 3692 | 58 |