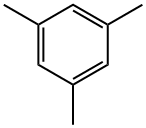
Мезитилен
- английское имяMesitylene
- CAS №108-67-8
- CBNumberCB5852758
- ФормулаC9H12
- мольный вес120.19
- EINECS203-604-4
- номер MDLMFCD00008538
- файл Mol108-67-8.mol
| Температура плавления | -45 °C |
| Температура кипения | 163-166 °C(lit.) |
| плотность | 0.864 g/mL at 25 °C(lit.) |
| плотность пара | 4.1 (vs air) |
| давление пара | 14 mm Hg ( 55 °C) |
| показатель преломления | n |
| Fp | 112 °F |
| температура хранения | 2-8°C |
| растворимость | Miscible with alcohol, benzene, ether (Windholz et al., 1983), and trimethylbenzene isomers. |
| пка | >14 (Schwarzenbach et al., 1993) |
| форма | Liquid |
| цвет | Clear colorless |
| Порог?обнаружения?запаха? | 0.17ppm |
| Пределы взрываемости | 0.88-6.1%, 100°F |
| Растворимость в воде | 2.9 g/L (20 ºC) |
| Мерк | 14,5907 |
| БРН | 906806 |
| констант закона Генри | 4.03, 4.60, 5.71, 6.73, and 9.63 at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth et al.,1988) |
| Пределы воздействия | NIOSH REL: TWA 25 ppm (125 mg/m3); ACGIH TLV: TWA for mixed isomers 25 ppm (adopted). |
| Диэлектрическая постоянная | 3.4(Ambient) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| ИнЧИКей | AUHZEENZYGFFBQ-UHFFFAOYSA-N |
| LogP | 3.42 at 20℃ |
| Справочник по базе данных CAS | 108-67-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 887L18KQ6X |
| Справочник по химии NIST | Benzene, 1,3,5-trimethyl-(108-67-8) |
| Система регистрации веществ EPA | 1,3,5-Trimethylbenzene (108-67-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xi,N,F,T | |||||||||
| Заявления о рисках | 10-37-51/53-39/23/24/25-23/24/25-11-37/38 | |||||||||
| Заявления о безопасности | 61-45-36/37-16-7 | |||||||||
| РИДАДР | UN 2325 3/PG 3 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 25 ppm (125 mg/m3) | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | OX6825000 | |||||||||
| F | 10 | |||||||||
| Температура самовоспламенения | 1022 °F | |||||||||
| Примечание об опасности | Irritant/Flammable | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29029080 | |||||||||
| Банк данных об опасных веществах | 108-67-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 (inhalation) for rats 24 g/m3/4-h (quoted, RTECS, 1985). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H411:Токсично для водных организмов с долгосрочными последствиями.
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H304:Может быть смертельным при проглатывании и последующем попадании в дыхательные пути.
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P331:немедленно всю загрязненную одежду. Промыть кожу водой. Не вызывать рвоту!
Мезитилен химические свойства, назначение, производство
Химические свойства
Mesitylene is a clear, colorless liquid. Distinctive, aromatic odor.Физические свойства
Colorless liquid with a peculiar odor. An odor threshold concentration of 170 ppbv was reported by Nagata and Takeuchi (1990).Использование
Mesitylene is used to make plastics and dyes. It acts as a solvent, ligand in organometallic chemistry and precursor to 2,4,6-trimethylaniline. It is also used as a developer for photopatternable silicones due to its solvent properties in the electronics industry. It is used as an internal standard in nuclear magnetic resonance (NMR) samples due to the presence of three equivalent protons in it. It is involved in the production of trimesic acid and antioxygen, epoxy firming agent and polyester resin stabilizers. Further, it is used as an additive and component in aviation gasoline blends.Определение
ChEBI: A trimethylbenzene carrying methyl substituents at positions 1, 3 and 5.Общее описание
A colorless liquid with a peculiar odor. Insoluble in water and less dense than water. Flash point near 123°F. May be toxic by ingestion and inhalation. Used to make plastics and dyes.Реакции воздуха и воды
Flammable. Insoluble in water.Профиль реактивности
TRIMETHYLBENZENE is incompatible with the following: Oxidizers, nitric acid .Опасность
Moderate fire hazard. Toxic by inhalation. Central nervous system impairment, asthma, and hematologic effects.Угроза здоровью
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.Пожароопасность
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.Профиль безопасности
Poison by inhalation. Moderately toxic by intraperitoneal route. Human systemic effects by inhalation: sensory changes involving peripheral nerves, somnolence (general depressed activity), and structural or functional change in trachea or bronchi. Reports of leukopenia and thrombocytopenia in experimental animals. A mild skin and eye irritant. A flammable liquid when exposed to heat or flame; can react vigorously with oxidizing materials. Violent reaction with HNO3. To fight fire, use water spray, fog, foam, CO2. Emitted from modern buildmg materials (CENEAR 69,22,91). When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
Mesitylene is used as raw material in chemical synthesis and as ultraviolet stabilizer; as a paint thinner, solvent, and motor fuel component; as an intermediate in organic chemical manufacture.Экологическая судьба
Biological. In anoxic groundwater near Bemidji, MI, 1,3,5-trimethylbenzene anaerobically biodegraded to the intermediate tentatively identified as 3,5-dimethylbenzoic acid (Cozzarelli et al., 1990).Photolytic. Glyoxal, methylglyoxal, and biacetyl were produced from the photooxidation of 1,3,5-trimethylbenzene by OH radicals in air at 25 °C (Tuazon et al., 1986a). The rate constant for the reaction of 1,3,5-trimethylbenzene and OH radicals at room temperature was 4.72 x 10-11 cm3/molecule?sec (Hansen et al., 1975). A rate constant of 2.97 x 10-8 L/molecule?sec was reported for the reaction of 1,3,5-trimethylbenzene with OH radicals in the gas phase (Darnall et al., 1976). Similarly, a room temperature rate constant of 6.05 x 10-11 cm3/molecule?sec was reported for the vapor-phase reaction of 1,3,5-trimethylbenzene with OH radicals (Atkinson, 1985). At 25 °C, a rate constant of 3.87 x 10-11 cm3/molecule?sec was reported for the same reaction (Ohta and Ohyama, 1985).
Chemical/Physical. 1,3,5-Trimethylbenzene will not hydrolyze because it does not contain a hydrolyzable functional group (Kollig, 1993).
Перевозки
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.Методы очистки
Dry it with CaCl2 and distil it from Na in a glass helices-packed column. Treat it with silica gel and redistil it. Alternative purifications include vapour-phase chromatography, or fractional distillation followed by azeotropic distillation with 2-methoxyethanol (which is subsequently washed out with H2O), drying and fractional distilling. More exhaustive purification uses sulfonation by dissolving in two volumes of conc H2SO4, precipitating with four volumes of conc HCl at 0o, washing with conc HCl and recrystallising from CHCl3. The mesitylene sulfonic acid is hydrolysed with boiling 20% HCl and steam distilled. The separated mesitylene is dried (MgSO4 or CaSO4) and distilled. It can also be fractionally crystallised from the melt at low temperatures. [Beilstein 5 IV 1016.]Несовместимости
Vapors forms explosive mixture with air. Violent reaction with nitric acid. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxidesУтилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.Мезитилен запасные части и сырье
Мезитилен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0316-2224727,2224728,2224729 +86-18631652130 |
China | 23 | 58 | ||
| +86-13806087780 | China | 17365 | 58 | ||
| +86-0519-85551759 +8613506123987 |
China | 8840 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-025-83346564; +8613182976929 |
China | 122 | 58 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7738 | 55 |
Мезитилен Обзор)
- 1,2,4,5-Тетраметилбензол
- 2,3,6-триметилфенола
- 2-Methylbenzotrifluoride
- Три-o-толилфосфин
- 1,3,5-Триметоксибензол
- 2-(трифторметил)бензальдегид
- 3-(Трифторметил)бензальдегид
- 3 - (трифторметил) бензойной кислоты
- Толилтриазол
- 2,3,5-Триметилфенол
1of4