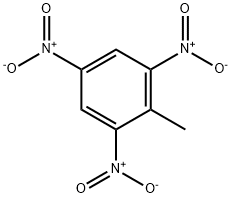
2,4,6-ТРИНИТРОТОЛУОЛ
- английское имя2,4,6-Trinitrotoluene
- CAS №118-96-7
- CBNumberCB0487625
- ФормулаC7H5N3O6
- мольный вес227.13
- EINECS204-289-6
- номер MDLMFCD00041875
- файл Mol118-96-7.mol
| Температура плавления | 80.9℃ |
| Температура кипения | 335–340°C |
| плотность | 1.654g/cm3 |
| давление пара | 0.2 at 20 °C (NIOSH, 1997)4.26 at 54.76 °C, 2.557 at 72.49 °C, 4.347 at 76.06 °C (Knuden effusion method, Lenchitz andVelicky, 1970) |
| показатель преломления | 1.5500 (estimate) |
| Fp | 2 °C |
| температура хранения | 2-8°C |
| растворимость | Solubility Very sparingly soluble in water; soluble in acetone, benzene; less soluble in ethanol |
| форма | crystals |
| цвет | Yellow |
| Водородный показатель | Colorless (11.5) to orange (14.0) |
| Растворимость в воде | 0.12g/L(20.0 ºC) |
| Пределы воздействия | TLV-TWA (skin) 0.5 mg/m3 (ACGIH and MSHA), 1.50 mg/m3 (OSHA). |
| Диэлектрическая постоянная | 2.2(20℃) |
| Стабильность | Unstable. Risk of explosion if heated or struck. Reacts violently - potentially explosively - with reducing agents. Reacts with heavy metals. |
| Основное приложение | Explosive, energetic materials, preparation of diamond |
| LogP | 1.65 at 20℃ |
| Справочник по базе данных CAS | 118-96-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4 |
| FDA UNII | H43RF5TRM5 |
| Предложение 65 Список | 2,4,6-Trinitrotoluene |
| МАИР | 3 (Vol. 65) 1996 |
| Система регистрации веществ EPA | Trinitrotoluene (118-96-7) |
| Коды опасности | E,T,N,Xn,F,B | |||||||||
| Заявления о рисках | 2-23/24/25-33-51/53-36-20/21/22-11-1 | |||||||||
| Заявления о безопасности | 35-45-61-36/37-26-16 | |||||||||
| РИДАДР | 0209 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.5 mg/m3 [skin] | |||||||||
| WGK Германия | 2 | |||||||||
| Класс опасности | 1.1D | |||||||||
| Банк данных об опасных веществах | 118-96-7(Hazardous Substances Data) | |||||||||
| Токсичность | Acute oral LD50 in mice 660 mg/kg, rats 795 (quoted, RTECS, 1985). | |||||||||
| ИДЛА | 500 mg/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H302+H312+H332:Вредно при проглатывании, при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
P403+P235:Хранить в прохладном, хорошо вентилируемом месте.
2,4,6-ТРИНИТРОТОЛУОЛ химические свойства, назначение, производство
Описание
2,4,6-Trinitrotoluene (TNT) is a yellow, odourless, unstable solid. TNT does not occur naturally in the environment. TNT is an explosive used in military shells, bombs, and grenades; in industrial uses; and in underwater blasting. TNT is a high explosive that is unaffected by ordinary shocks and therefore must be set off by a detonator. TNT is often mixed with other explosives such as ammonium nitrate to form amatol. Because it is insensitive to shock and must be exploded with a detonator, it is the most favoured explosive used in munitions and construction. TNT reacts violently, is potentially explosively, reacts with heavy metals, and is a chemical with risk of explosion if heated or struck.Химические свойства
TNT exists in five isomers; 2,4,6-trinitrotoluene is the most commonly used. It is a colorless to pale yellow odorless solid (pellets, cast blocks, and cast slabs) or crushed flakes.Физические свойства
Colorless to light yellow, odorless monoclinic crystals. Soluble in alcohol and ether; insoluble in water.Использование
2,4,6-Trinitrotoluene (TNT) is used as a high explosive in mining and in military. It is produced by nitration of toluene with a mixture of nitric and sulfuric acids.Определение
ChEBI: 2,4,6-trinitrotoluene is a trinitrotoluene having the nitro groups at positions 2, 4 and 6. It has a role as an explosive. It is functionally related to a 1,3,5-trinitrobenzene.Общее описание
A slurry of a yellow water-insoluble crystalline solid. Can burn, although difficult to ignite. When water has been driven off or evaporated the residue is easily ignited, burns vigorously, and is highly explosive . Produces toxic oxides of nitrogen during combustion. May explode under exposure to intense heat. Primary hazard is blast of an explosion, not flying projectiles or fragments.Профиль реактивности
TRINITROTOLUENE may begin a vigorous reaction that culminates in a detonation if mixed with reducing agents, including hydrides, sulfides and nitrides. May explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents.Угроза здоровью
Some are toxic and may be fatal if inhaled, swallowed or absorbed through skin. Contact may cause burns to skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.Пожароопасность
TNT is a high explosive. In comparison to many other high explosives, it is insensitive to heat, shock, or friction. Small amounts may burn quietly without detonation. However, when heated rapidly or subjected to strong shock, it detonates. Its detonation temperature is 470°C (878°F) and its velocity is between 5.1 and 6.9 km/s. In combination with other explosives, TNT is widely used as a military and industrial explosive. Amatol, cyclonite, and tetrytol are some of the examples of such explosive combinations. Amatol is a composition of 80% ammonium nitrate and 20% TNT by mass. TNT itself has a very high brisance.Products from the detonation of 1.5-2.0 kg of TNT in air- and oxygen-deficient atmospheres consisted of low-molecularweight gases and high-molecular-weight polycyclic aromatic hydrocarbons (Johnson et al. 1988). Greiner and associates (1988) examined the soots produced from the detonation of cast composites of TNT mixed with nitroguanidine or RDX in 1 atmosphere of argon. The soot contained 25 wt% diamond 4-7 nm in diameter, the IR spectrum and particle size of which resembled those from meteorites. .
Возможный контакт
TNT is used as an explosive, that is, as a bursting charge in military explosive shells, bombs, grenades, and mines; and an intermediate in dyestuffs and photographic chemicals.Канцерогенность
In bacterial and mammalian in vitro cell systems TNT is a direct-acting mutagen. However, inclusion of exogenous metabolic activation appears to abolish the genotoxicity. In vivo assays of TNT have not shown it to be genotoxic, suggesting that TNT may be reduced to nonmutagenic metabolic products in the whole animal.хранилище
TNT is stored in a permanent magazine, separated from combustible and oxidizable materials, initiators, and heat sources. It is shipped in amounts not exceeding 60 lb (27 kg) in weight in metal containers enclosed in wooden or fiberboard boxes.Перевозки
UN1356 Trinitrotoluene, wetted with not <30% water, by mass, Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN0209 Trinitrotoluene or TNT, dry or wetted with < 30% water, by mass, Hazard Class: 1D; Labels:1DExplosive (with a mass explosion hazard); D-Substances or articles which may mass detonate (with blast and/or fragment hazard) when exposed to fire.Методы очистки
Crystallise TNT from *benzene and EtOH. Then fuse (CARE) and allow to crystallise under vacuum. Gey, Dalbey and Van Dolah [J Am Chem Soc 78 1803 1956] dissolved TNT in acetone and added cold water (1:2:15), the precipitate was filtered off, washed free from solvent and stirred with five parts of aqueous 8% Na2SO3 at 50-60o for 10minutes. This was filtered, washed with cold water until the effluent was colourless, and air dried. The product was dissolved in five parts of hot CCl4, washed with warm water until the washings were colourless and TNT was recoverd by cooling and filtering. It was recrystallised from 95% EtOH and carefully dried over H2SO4. The dry solid should not be heated without taking precautions for a possible EXPLOSION. Work with small quantities. [Beilstein 5 H 347, 5 I 172, 5 II 268, 5 III 767, 5 IV 873.]Несовместимости
Sensitive to shock and heat. Incompatible with initiating explosives, combustible materials. Aromatic nitro compounds, such as trinitrobenzene, range from slight to strong oxidizing agents. Keep away from strong reducing agents, including hydrides, alkali metals; aluminium and other metal powder; phosphorus; sulfides and nitrides, alkaline material, strong bases; contact may initiate vigorous reactions that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions.Утилизация отходов
TNT is dissolved in acetone and incinerated. The incinerator should be equipped with an afterburner and a caustic soda solution scrubber.2,4,6-ТРИНИТРОТОЛУОЛ запасные части и сырье
запасной предмет