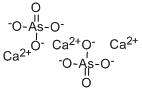
АРСЕНАТ КАЛЬЦИЯ
- английское имяCALCIUM ARSENATE
- CAS №7778-44-1
- CBNumberCB5671562
- ФормулаAsH3O4.3/2Ca
- мольный вес398.07
- EINECS231-904-5
- номер MDLMFCD00049407
- файл Mol7778-44-1.mol
| Температура плавления | 1455℃ [CRC10] |
| плотность | 3,62 g/cm3 |
| давление пара | 0Pa at 20℃ |
| RTECS | CG0830000 |
| растворимость | soluble in dilute acid solutions |
| форма | Powder |
| цвет | White |
| Удельный вес | 3.62 |
| Растворимость в воде | It is soluble in water and acids, insoluble in organic solvents |
| Константа произведения растворимости (Ksp) | pKsp: 18.17 |
| Пределы воздействия | ACGIH: TWA 0.01 mg/m3 NIOSH: IDLH 5 mg/m3; Ceiling 0.002 mg/m3 |
| Справочник по базе данных CAS | 7778-44-1(CAS DataBase Reference) |
| FDA UNII | 95OX15I8ZU |
| Система регистрации веществ EPA | Calcium arsenate (7778-44-1) |
| Заявления о рисках | 23/25-45-50/53 | |||||||||
| Заявления о безопасности | 45-53 | |||||||||
| OEL | Ceiling: 0.002 mg/m3 [15-minute] | |||||||||
| РИДАДР | 1573 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28429090 | |||||||||
| Банк данных об опасных веществах | 7778-44-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in female rats: 298 mg/kg (Gaines) | |||||||||
| ИДЛА | 5 mg As/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
вредная бумага
H301:Токсично при проглатывании.
H350:Может вызывать раковые заболевания.
H400:Чрезвычайно токсично для водных организмов.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H331:Токсично при вдыхании.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P405:Хранить в недоступном для посторонних месте.
АРСЕНАТ КАЛЬЦИЯ химические свойства, назначение, производство
Описание
The calcium arsenate that is sold commercially today as an insecticide is not a single chemical compound but a complex mixture of several calcium arsenates and an excess of calcium hydroxide. The material is made from arsenic trioxide by first oxidizing it to arsenic pentoxide with nitric acid and then reacting the solution of arsenic pentoxide or arsenic acid with a slurry of calcium hydroxide. The conditions of temperature,concentration, and duration of reaction are important because of their influence on the physical nature of the product.Химические свойства
White powder. Slightly soluble in water; soluble in dilute acids. Decomposes on heating.Физические свойства
Commercial calcium arsenate generally is colored pink and is alkaline in reaction. It is a finely divided powder.Использование
Calcium arsenate has been used extensively against certain insects affecting field crops, especially cotton. It cannot be used safely on apples, peaches, beans, and some other crops because of its burning effect on the foliage and fruit. It is banned by a number of countries.Подготовка
An old method involving electrolysis in solution for the manufacture of calcium arsenate is as follows. A solution of arsenious oxide in caustic soda (As2O3:NaOH=198:250) is electrolyzed between iron electrodes. Hydrogen and a small quantity of metallic arsenic are liberated at the cathode and very little oxygen at the anode, the basic arsenite in solution being oxidized to arsenate. When this oxidation is complete, any arsenic is filtered off and the solution treated with milk of lime. A basic arsenate of extremely low solubility is precipitated and after removal, is washed and dried.Общее описание
White powder. Slightly soluble in water. Toxic by inhalation and ingestion. Used as an insecticide and germicide.Реакции воздуха и воды
Slightly soluble in water.Профиль реактивности
Has only weak oxidizing power but redox reactions can still occur. Soluble in dilute acids. [Hawley]. Produces toxic fumes of arsenic when heated to decomposition .Опасность
Toxic by ingestion and inhalation.Угроза здоровью
CALCIUM ARSENATE is extremely toxic; the probable oral lethal dose for humans is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 150 lb. person. It is an irritant to eyes, respiratory tract, mouth and stomach. Damage to kidneys, liver and the nervous system have been reported. (Non-Specific -- Arsenic) Chronic exposure can cause bone marrow damage, often leading to aplastic anemia. There is epidemiological evidence that chronic ingestion of arsenic compounds causes a predisposition to skin cancers.Пожароопасность
Fire may produce irritating or poisonous gases. When heated to decomposition, CALCIUM ARSENATE produces toxic fumes of arsenic. Avoid heat. Hazardous polymerization may not occur.Профиль безопасности
Confirmed human carcinogen. Poison by ingestion. Moderately toxic by skin contact. When heated to decomposition it emits toxic fumes of arsenic.АРСЕНАТ КАЛЬЦИЯ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 | |
| +86-852-30606658 | China | 43340 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 86-21-51086038 | CHINA | 23912 | 58 | |
| 400-6106006 | China | 30123 | 84 | |
| 0370-2059268 17550075363 |
China | 2283 | 58 |