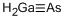
Галлий арсенид
- английское имяGallium arsenide
- CAS №1303-00-0
- CBNumberCB7249244
- ФормулаAsGa
- мольный вес144.64
- EINECS215-114-8
- номер MDLMFCD00011017
- файл Mol1303-00-0.mol
| Температура плавления | 1238°C |
| плотность | 5.31 g/mL at 25 °C (lit.) |
| давление пара | 0Pa at 20℃ |
| показатель преломления | 3.57 |
| форма | pieces |
| цвет | Dark gray |
| Удельный вес | 5.31 |
| удельное сопротивление | ≥1E7 Ω-cm |
| Растворимость в воде | Soluble in hydrochloric acid. Insoluble in water, ethanol, methanol and acetone. |
| Полупроводниковые характеристики | <100> |
| Кристальная структура | Cubic, Sphalerite Structure - Space Group F(-4)3m |
| Мерк | 14,4347 |
| Справочник по базе данных CAS | 1303-00-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 8 |
| FDA UNII | 27FC46GA44 |
| Предложение 65 Список | Gallium Arsenide |
| МАИР | (Vol. 86, 100C) 2012 |
| Система регистрации веществ EPA | Gallium arsenide (1303-00-0) |
| UNSPSC Code | 12352300 |
| NACRES | NA.23 |
| Коды опасности | T,N | |||||||||
| Заявления о рисках | 23/25-50/53-48/23-45 | |||||||||
| Заявления о безопасности | 20/21-28-45-60-61-53 | |||||||||
| РИДАДР | UN 1557 6.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | LW8800000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 2853909090 | |||||||||
| Банк данных об опасных веществах | 1303-00-0(Hazardous Substances Data) | |||||||||
| Токсичность | mouse,LD50,intraperitoneal,4700mg/kg (4700mg/kg),BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE)BEHAVIORAL: FOOD INTAKE (ANIMAL),Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 45(10), Pg. 13, 1980. | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H350:Может вызывать раковые заболевания.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H360F:Может отрицательно повлиять на способность к деторождению.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Галлий арсенид химические свойства, назначение, производство
Химические свойства
Gallium arsenide contains 48.2% gallium and 51.8% arsenic and is considered an intermetallic compound. It occurs as cubic crystals with a dark gray metallic sheen. Gallium arsenide is electroluminescent in infrared light. Gallium arsenide readily reacts with oxygen in air, forming a mixture of oxides of gallium and arsenic on the crystal surface. Gallium arsenide presents the following properties: hardness, 4.5; thermal expansion coefficient, 5.9×106; thermal conductivity, 0.52Wunits; specific heat, 0.086 cal/g/ ℃; intrinsic electron concentration, 107; energy gap at room temperature, 1.38 eV; electron mobility, 8800 cm2/V/s; effective mass for electrons, 0.06m0; lattice constant,5.6–54 AO; dielectric constant, 11.1; intrinsic resistivity at 300K=3.7×108Ωcm; electron lattice mobility at 300K= 10,000 cm2/V/s; intrinsic charge density at 300K=1.4 106 cm-3; electron diffusion constant at 300K=310 cm2/s; and hole diffusion constant=11.5 cm2/s.
Garlic odor when moistened. Finely divided gallium arsenide can react vigorously with steam, energetic acids, and oxidizers to evolve arsine gas, and can release arsenic fumes when heated to decomposition. The molten form attacks quartz.
Физические свойства
Gray cubic crystal; density 5.316 g/cm3; melts at 1,227°C; hardness 4.5 Mohs; lattice constant 5.653?; dielectric constant 11.1; resistivity (intrinsic) at 27°C, 3.7x108 ohm-cm.Использование
In semiconductor applications (transistors, solar cells, lasers).Gallium arsenide is among the most widely used intermetallic semiconductor components (Harrison, 1986; McIntyr and Sherin, 1989). Gallium arsenide is also incorporated into light-emitting diodes and photovoltaic cells, while gallium alloys are used for dental amalgam as a low toxicity replacement for mercury.Подготовка
Gallium arsenide is prepared by passing a mixture of arsenic vapor and hydrogen over gallium(III) oxide heated at 600°C: Ga2O3 + 2As + 3H2 2GaAs + 3H2OThe molten material attacks quartz. Therefore, quartz boats coated with carbon by pyrolytic decomposition of methane should be used in refining the compound to obtain high purity material. Gallium arsenide is produced in polycrystalline form as high purity, single crystals for electronic applications. It is produced as ingots or alloys, combined with indium arsenide or gallium phosphide, for semiconductor applications.Общее описание
Dark gray crystals with a metallic greenish-blue sheen or gray powder. Melting point 85.6°F (29.78°C).Реакции воздуха и воды
Stable in dry air. Tarnishes in moist air. Insoluble in water.Профиль реактивности
GALLIUM ARSENIDE can react with steam, acids and acid fumes. Reacts with bases with evolution of hydrogen. Attacked by cold concentrated hydrochloric acid. Readily attacked by the halogens. The molten form attacks quartz.Опасность
Toxic metal. Questionable carcinogenПожароопасность
Flash point data for GALLIUM ARSENIDE are not available; however, GALLIUM ARSENIDE is probably combustible.Профиль безопасности
Confirmed carcinogen. Mddly toxic by intraperitoneal route. Most arsenic compounds are poisons. Can react with steam, acids, and acid fumes to evolve the deadly poisonous arsine. Molten gallium arsenide attacks quartz. When heated to decomposition it emits very toxic fumes of As. See also ARSENIC COMPOUNDS and GALLIUM COMPOUNDS.Канцерогенность
Carcinogenesis.Gallium is antineoplastic in several human and murine cancer cell lines and in some in vivo cancers. It has been used experimentally in patients in the treatment of lymphatic malignancies (including multiple myelomas) and for urothelial malignancies. However, the dissociation of gallium arsenide into gallium and arsenic is a factor to take into account. A considerable number of studies have evaluated the carcinogenic potential of arsenic and various arsenic compounds.
Структура и конформация
The space lattice of gallium arsenide (GaAs) belongs to the cubic system Td2, and its zincblende-type structure has a lattice constant of a=0.5654 nm and a distance to its nearest neighbor of 0.244 nm.Галлий арсенид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8615250961469 | China | 9720 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +8618058761490 | China | 49983 | 58 | |
| +1-+1(833)-552-7181 | United States | 57505 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 |