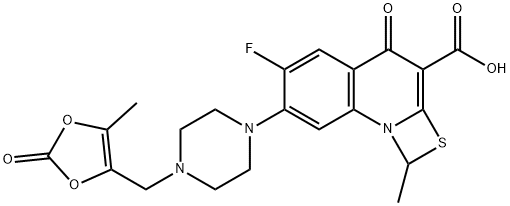
Прулифлоксацин
- английское имяPrulifloxacin
- CAS №123447-62-1
- CBNumberCB5474525
- ФормулаC21H20FN3O6S
- мольный вес461.46
- EINECS819-932-1
- номер MDLMFCD00864847
- файл Mol123447-62-1.mol
химическое свойство
| Температура плавления | 211-214°C |
| Температура кипения | 633.2±65.0 °C(Predicted) |
| плотность | 1.62±0.1 g/cm3(Predicted) |
| температура хранения | Sealed in dry,Store in freezer, under -20°C |
| растворимость | 1 M NaOH: soluble25ML, clear, colorless (Solvent: 1 mg + 25 mL of NaOH) |
| пка | 5.85±0.40(Predicted) |
| форма | White to yellow crystalline solid. |
| цвет | Off-White to Pale Yellow |
| λмакс | 276nm(H2O)(lit.) |
| Мерк | 14,7908 |
| Справочник по базе данных CAS | 123447-62-1(CAS DataBase Reference) |
| FDA UNII | J42298IESW |
| Код УВД | J01MA17 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Прулифлоксацин химические свойства, назначение, производство
Описание
Prulifloxacin was launched as the third fluoroquinone. It was introduced in Japan as an oral treatment for urinary tract infections (UTls), respiratory tract infections (RTls) and bacterial pneumoniae. It can be synthesized in 10 steps from commercially available 3,4-difluoroaniline. Key steps involve the cyclization of 6,7-difluoro-rl-hydroxy-2- thioquinoline-3carboxylic acid ethyl ester with 1 ,I-dibromomethane to give the corresponding thiazeto-[3,2a]quinoline. Aromatic nucleophilic substitution of the 7-fluoro atom with piperazine followed by hydrolysis of the ethyl ester and finally alkylation of the piperazinyl moiety with 4-(bromomethyl)-5-methyl-l ,bdioxol-Bone complete the synthesis. Prulifloxacin is a lipophilic prodrug, which is rapidly hydrolyzed to the corresponding Ndealkylated piperazine, NM 394, by paraoxonase type enzymes in blood and liver following intestinal absorption. The DNA gyrase inhibitor NM 394 accounts for all antimicrobial activity: it shows a similar or greater activity against gram-positive bacteria compared to ciprofloxacin, and a greater activity in the case of gram-negative bacteria. In clinical studies, prulifloxacin has shown good efficacy against UTls and RTls. The drug is mainly excreted in the urine and in the feces as unchanged NM 394, which has a plasma half-life of approximately 8 h. Phototoxicity in animal models is less severe than with other quinolones. Prulifloxacin is well tolerated with an adverse effect profile similar to that of other fluoroquinolones.Химические свойства
Off-White SolidИспользование
Prulifloxacin is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class. Prulifloxacin is a prodrug for active metabolite, Ulifloxacin. Antibacterial.Фармацевтические приложения
A lipophilic prodrug which is very rapidly metabolized by esterase into ulifloxacin, a 6-fluoro, 7-piperazinyl thiazetoquinoline.Ulifloxacin is moderately active against Staph. aureus (MIC 0.4–0.8 mg/L) and inactive against Str. pneumoniae (MIC 2–8 mg/L) as well as against Enterococcus spp. Against Enterobacteriaceae (MIC 0.05–0.8 mg/L) and Ps. aeruginosa (MIC 0.2–0.8 mg/L) activity is similar to that of ciprofloxacin. It is active against fastidious Gram-negative bacilli, but not against anaerobes and non-fermentative Gram-negative bacilli. Activity against Acinetobacter spp. is modest.
Prulifloxacin is rapidly converted into ulifloxacin and after 3 h is no longer detected in blood. In volunteers receiving a single oral dose, peak plasma concentrations of 0.68 mg/L (300 mg dose) to 1.88 mg/L (for 400 mg dose) were attained between 0.67 and 1.25 h. The mean apparent elimination half-life was 8 h and the mean cumulative elimination rate in urine within 48 h was 31–46%. Other inactive metabolites account for 7% of the dose. Half the administered dose is eliminated in feces within 72 h as ulifloxacin and 4% as prulifloxacin. Protein binding is 45%.
Прулифлоксацин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 17791478691 | CHINA | 296 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| +8619521488211 | China | 2558 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13806087780 | China | 17365 | 58 |