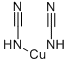
Cupric cyanide
- русский язык имя
- английское имяCupric cyanide
- CAS №14763-77-0
- CBNumberCB2851871
- ФормулаC2CuN2
- мольный вес115.5808
- EINECS238-826-0
- номер MDLMFCD01745912
- файл Mol14763-77-0.mol
химическое свойство
| Температура плавления | decomposes [CRC10] |
| Температура кипения | 41°C (estimate) |
| растворимость | insoluble in H2O; soluble in acid solutions, alkaline solutions |
| форма | green powder |
| цвет | green |
| Растворимость в воде | insoluble H2O; soluble acids and alkalies [HAW93] |
| FDA UNII | Y10612MK1F |
| Система регистрации веществ EPA | Copper cyanide (Cu(CN)2) (14763-77-0) |
Cupric cyanide химические свойства, назначение, производство
Химические свойства
Cupric cyanide is a yellowish-green powder which decomposes on heating.Опасность
Toxic.Профиль безопасности
Poison by intraperitoneal route. See also CYANIDE and COPPER COMPOUNDS. Incompatible with magnesium. When heated to decomposition it emits toxic fumes of NOx and CN-.Возможный контакт
Copper cyanide is used in electroplating copper on iron; and as an insecticide and a catalyst.Несовместимости
Contact with heat, strong acids (HCl, H2SO4, HNO3) forms deadly hydrogen cyanide gas. May release hydrogen cyanide on contact with moisture. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acetylene gas, and chemically active metals, such as potassium, sodium, magnesium, and zinc.Утилизация отходов
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill. Copper-containing wastes can be concentrated to the point where copper can be electrolytically removed and reclaimed. If recovery is not feasible, the copper can be precipitated by alkali; the cyanide destroyed by alkaline oxidation yielding a sludge which can be sent to a chemical waste landfill. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.Cupric cyanide поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 |