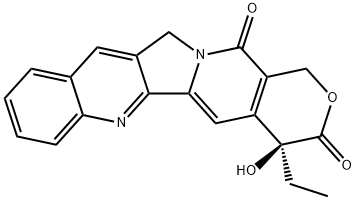
(+)-камpдоthecin
- английское имя(+)-Camptothecin
- CAS №7689-03-4
- CBNumberCB2381608
- ФормулаC20H16N2O4
- мольный вес348.35
- EINECS444-280-6
- номер MDLMFCD00081076
- файл Mol7689-03-4.mol
| Температура плавления | 260 °C (dec.)(lit.) |
| альфа | D25 +31.3° (in chloroform-methanol, 8:2) |
| Температура кипения | 482.73°C (rough estimate) |
| плотность | 1.3112 (rough estimate) |
| показатель преломления | 1.5700 (estimate) |
| температура хранения | 2-8°C |
| растворимость | chloroform/methanol (4:1): 4 mg/mL |
| форма | solid |
| пка | pKa 10.83 (Uncertain) |
| цвет | yellow |
| Растворимость в воде | insoluble |
| Мерк | 14,1735 |
| БРН | 631069 |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| ИнЧИКей | VSJKWCGYPAHWDS-FQEVSTJZSA-N |
| LogP | 1.740 |
| Справочник по базе данных CAS | 7689-03-4(CAS DataBase Reference) |
| Словарь онкологических терминов NCI | camptothecin |
| FDA UNII | XT3Z54Z28A |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
| Коды опасности | T,Xi,Xn | |||||||||
| Заявления о рисках | 36/37/38-25-20/21/22 | |||||||||
| Заявления о безопасности | 45-36/37/39-26-36 | |||||||||
| РИДАДР | UN 1544 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | UQ0492000 | |||||||||
| Примечание об опасности | Irritant | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | Ⅲ | |||||||||
| кода HS | 29399990 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H340:Может вызывать генетические дефекты.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P405:Хранить в недоступном для посторонних месте.
(+)-камpдоthecin химические свойства, назначение, производство
Описание
Camptothecin is an alkaloid derived from Xi Shu (Camptotheca acuminata), which belongs to Nyssaceae. The traditional Chinese medicine Camptotheca acuminata (Xi Shu) has been collected in the Compilation of Chinese Herbal Medicine, Chinese Materia Medica, and Great Dictionary of Chinese Medicine. Camptotheca acuminata (Xi Shu) is widely distributed in the basin of Yangtze river and the southwestern provinces. The main medicinal parts of Camptotheca acuminata (Xi Shu) are root bark and fruit, which get rid of heat and toxic materials and eliminate the disease.Химические свойства
light yellow crystal powdeФизические свойства
Appearance: pale yellow needlelike crystal. Solubility: slightly soluble in ethanol and chloroform; poorly soluble in water; camptothecin fails to generate stable salt with acid, whereas it can produce sodium salt which is soluble in water by reacting with heated sodium hydroxide solution. Melting point: 264–267?°C. Camptothecin derivativesИстория
In 1966, Wall M E et? al. from the United States isolated an alkaloid from Camptotheca acuminata and defined its chemical structure. The in?vitro anticancer tests revealed the anticancer activity of the tryptophan-terpene alkaloid, which is known as camptothecin and received widely concern. In 1975, Corey et?al. first opened the door for the chiral synthesis of camptothecin, but the reaction step was long and the yield rate was very low. It was not until 1997 that Ciufolini et?al. developed a new method for the synthesis of camptothecin by five steps, with a total yield rate up to 51%. The great breakthrough in the chemical synthesis of camptothecin has made its extensive application become a reality.Hydroxycamptothecin, as a camptothecin derivative with a hydroxyl group on the tenth carbon atom, is widely used for the treatment of various cancers. In 1969, researchers from Shanghai Institute of Materia Medica found that hydroxycamptothecin possessed potent anticancer activity and low toxicity. And this finding promoted the production and clinical application of hydroxycamptothecin, but its usage was interrupted for technology and quality. In the 1980s, hydroxycamptothecin was reproduced for clinical application with an improvement in producing technology, and hydroxycamptothecin got its approval number in 1986 for clinical usage in China. In the 1990s, the US Food and Drug Administration approved the clinical application of topotecan and irinotecan, which played a significant role in the prevention and treatment of cancers.
Использование
Antitumor alkaloid. Binds irreversible to the DNA-topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. A cytotoxic antitumor agentПоказания
It is mainly used in digestive tract tumors and has a good effect on gastric cancer, rectal cancer, and colon cancer. Besides, it can improve the surgical resection of advanced gastric cancer and also has some therapeutic effect on bladder cancer and lung adenocarcinoma. Moreover, camptothecin can be used for treatment of psoriasis, warts, acute and chronic leukemia, and hepatosplenomegaly caused by schistosomiasis.Определение
ChEBI: A pyranoindolizinoquinoline that is pyrano[3',4':6,7]indolizino[1,2-b]quinoline which is substituted by oxo groups at positions 3 and 14, and by an ethyl group and a hydroxy group at position 4 (the S enantiomer).Биологическая активность
Cytotoxic plant alkaloid with antitumor properties; prototypic DNA topoisomerase I inhibitor.Фармаколо?гия
The pharmacology of camptothecin was mainly manifested as antitumor activity. Camptothecin specifically targeted topoisomerase I and exerted anticancer activity by inhibiting the synthesis of DNA.?Camptothecin mainly influenced the S phase of cell cycle and was considered as a specific inhibitor agent of cell cycle. The results of animal experiments showed that camptothecin had some inhibitory effects on leukemia, Yoshida sarcoma, and Ehrlich ascites carcinoma.Previous clinical trials showed that camptothecin and its analogs have therapeutic effects on bladder cancer, brain cancer, breast cancer, cervical cancer, colon cancer, neural stromal tumor, lymphoreticulosis, lung cancer, leukemia, lymphoma, melanoma, ovarian cancer, pancreatic cancer, pediatric cancer, prostate cancer, and liver cancer.
Injection of camptothecin (2.5?mg/ml, 5–10?mg/day) with a treatment course of 140?mg achieved effective rate of 44.8% and 38.3% for gastric cancer and colon cancer, respectively. Hydroxycamptothecin can be used for the prevention and treatment of gastric, liver, head, and neck cancer and leukemia, and the effective rate is 44%. In addition, the dimethyl sulfoxide solution of camptothecin was also successfully used for treatment of psoriasis.
разработк И противоопухолевых препаратов
Camptothecin (CPT) is a monoterpene indole alkaloid which is isolatedfrom the Chinese plant, Camptotheca acuminata (Nyssaceae) (Wall et al. 1966).CPT is used in cancer treatment since it is a potent inhibitor of DNA topoisomeraseI, which leads to DNA damage and the apoptosis in cancer cells. Studies have shownthat CPT itself is not suitable for clinical application since it has low water solubilityand certain side effects; therefore, water-soluble CPT derivatives such as topotecan and irinotecan were synthesized and have been successfully used for thetreatment of ovarian, lung, and colorectal cancers, and CPT has been approved by theFood and Drug Administration (FDA) of the USA. Currently, topotecan and irinotecanare all synthesized from natural camptothecin which is mainly extracted fromCamptotheca acuminata (Beegum et al. 2007). Subsequently, CPT was also recognizedand extracted from other plant species such as Ervatamia heyneana (Gunasekeraet al. 1979), Melliodendron megacarpum (Arisawa et al. 1981), Nothapodytes foetida(Govindachari and Viswanathan 1972), and Ophiorrhiza species (Beegum et al.2007). However, the extraction of CPT from plants is limited because of low yields(about 1 mg/g dry weight) and scanty natural resources (Lopez-Meyer et al. 1994),and scientists have used biotechnological ways especially cell culture methods forthe production of CPT and its derivatives (Kai et al. 2015).Клиническое использование
Because of the toxicity and side effects of camptothecin, the currently used agents in clinical applications are camptothecin derivatives like topotecan, irinotecan, and hydroxycamptothecin. Topotecan, a water-soluble camptothecin derivative developed by SmithKline Beecham, was approved by FDA in 1996 for the treatment of ovarian cancer. As another water-soluble camptothecin derivative approved by FDA in 1996, irinotecan was mainly used in the treatment of advanced colorectal cancer. In addition, it was also shown to have obvious inhibitory effect on small cell lung cancer and leukemia. Hydroxycamptothecin possesses a broad-spectrum antitumor activity and was clinically used for intravesical therapy of bladder cancer. In addition, it has remarkable curative effect on colon cancer, breast cancer, gastric cancer, and leukemia.(+)-камpдоthecin запасные части и сырье
(+)-камpдоthecin поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618523575427 | China | 49732 | 58 | ||
| +86-13608205856; +undefined13608205856 |
China | 127 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7782 | 55 |