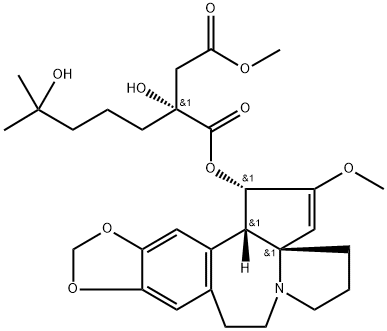
Homoharringtonine
- русский язык имя
- английское имяHomoharringtonine
- CAS №26833-87-4
- CBNumberCB2278852
- ФормулаC29H39NO9
- мольный вес545.63
- номер MDLMFCD05618221
- файл Mol26833-87-4.mol
| Температура плавления | 144-146 C |
| Температура кипения | 619.03°C (rough estimate) |
| плотность | 1.2395 (rough estimate) |
| показатель преломления | 1.6290 (estimate) |
| температура хранения | 2-8°C |
| растворимость | DMSO: soluble20mg/mL, clear |
| пка | 11.60±0.29(Predicted) |
| форма | powder |
| цвет | white to beige |
| оптическая активность | [α]/D -120 to -140°, c = 1 in chloroform-d |
| ИнЧИКей | DJIVDDPFKDEQIR-XSEHADPMSA-N |
| Словарь онкологических терминов NCI | homoharringtonine; omacetaxine mepesuccinate |
| FDA UNII | 6FG8041S5B |
| Словарь наркотиков NCI | omacetaxine |
| Код УВД | L01XX40 |
| Коды опасности | T+,Xi | |||||||||
| Заявления о рисках | 26/27/28-36/37/38-28 | |||||||||
| Заявления о безопасности | 36/37/39-45-27-26-36/37-28 | |||||||||
| РИДАДР | UN 1544 6.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | FK0260000 | |||||||||
| Класс опасности | 6.1(a) | |||||||||
| Группа упаковки | II | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H300+H310+H330:Смертельно при проглатывании, при контакте с кожей или при вдыхании.
-
оператор предупредительных мер
P262:Избегать попадания в глаза, на кожу или одежду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310+P330:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P310:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Немедленно обратиться за медицинской помощью.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
Homoharringtonine химические свойства, назначение, производство
Описание
Omacetaxine mepesuccinate (also known as homoharringtonine) was approved by the US FDA in October 2012 for the treatment of patients with chronic or accelerated phase chronic myeloid leukemia (CML) with resistance or intolerance to at least two tyrosine kinase inhibitors (TKIs). Omacetaxine is a protein synthesis inhibitor that was studied in the 1970s for the treatment of acute myeloid leukemia (AML) and in the 1990s for CML. Emergence of resistance to first- and second-generation TKIs has lead to renewed interest in omacetaxine due to its differentiated mode of action. Omacetaxine acts on the initial step of protein translation andresults in the rapid loss of a number of short-lived proteins that regulate proliferation and cell survival. Omacetaxine induces apoptosis and shows in vitro activity in anumberof leukemia cell lines andinmurine leukemiamodels. Omacetaxineis a naturally occurring alkaloid isolated from Cephalotaxus coniferous shrubs that are indigenous to Asia. Extracts of the bark have been used by practitioners of traditional Chinese medicine for the treatment of cancer. Although omacetaxine could be isolated directly from bark and roots, a more efficient approach is semi-synthesis by esterification of the abundant biosynthetic precursor cephalotaxine, which can be extracted from leaves rather than nonrenewable sources. Esterification is carried out with an activated ester in which the diol side-chain is protected as a tetrahydropyran; after ester formation, the diol is released in two steps under mild conditions.Химические свойства
Off-white CrystФизические свойства
Appearance: an almost white or pale yellow crystalline powder or an amorphous friable solid. It has the hygroscopic nature. It darkens on exposure to light. Solubility: easily soluble in chloroform, ethanol and methanol, and slightly soluble in ether and water. Melting point: 143–147?°C.История
In China, especially in Fujian, etc., doctors have treated tumor with Cephalotaxus harringtonia long ago. In the early 1970s, American scientists Powell et?al. isolated and identified the alkaloids of the Cephalotaxus plant and studied its antitumor activity. One type of the alkaloids (Cephalotaxine ester, including harringtonine, homoharringtonine, isoharringtonine, deoxyharringtonine, and pseudodeoxyharringtonine) was found to have the effect of inhibiting the proliferation of mouse leukemia cells . Chinese scientists also isolated a large number of harringtonine and homoharringtonine and used them to treat leukemia. Then, the United States and other developed countries’ scientists carried out the Phase I and II clinical research and toxicology research of homoharringtonine.Использование
Homoharringtonine (HHT) combined with some botanical drugs could induce cancer cells to resemble normal cells. HHT was prepared by a semi-synthetic method from Cephalotaxine, a major alkaloid of Cepahlotaxus species through the formation of a-ketoesОпределение
ChEBI: A cephalotaxine-derived alkaloid ester obtained from Cephalotaxus harringtonia; used for the treatment of chronic or accelerated phase chronic myeloid leukaemia.Показания
This product is recorded in the Pharmacopoeia of the People’s Republic of China (2015).Its main dosage form is homoharringtonine injection, which is mainly used for the treatment of chronic myelocytic leukemia and acute myeloid leukemia.
Биологическая активность
Inhibitor of protein synthesis. Blocks elongation phase of translation by binding to the 60-S ribosome subunit. Antileukemic.Фармаколо?гия
Induce apoptosis: Homoharringtonine was found to activate HL-60 cell apoptosis through caspase-3 mediated Bcl-2-Bax, -MAPK pathway .3. Induce cell differentiation: Homoharringtonine could induce HL-60 cell differentiation by downregulating the expression of CD44 gene, thereby increasing p27 and p21 expression and inhibition of cyclin E activity.
Structure-Activity Relationship (SAR) The methyl acetate group of 2′ position in the C-3 acyl side chain is an active essential group. R group plays a role in regulating the polarity of the molecule, and the size of the R group can affect the molecular activity. The olefinic carbon instead of the 2′ position chiral carbon is still active .
Клиническое использование
Synribo® (Omacetaxine mepesuccinate) was approved by the FDA for the treatment of adult patients with chronic or accelerated phase chronic myeloid leukemia (CML) exhibiting resistance or intolerance to tyrosine kinase inhibitors (TKI’s). Omacetaxine mepesuccinate inhibits protein synthesis and prevents aminoacyl-tRNA binding during the elongation phase and targets myeloma-promoting molecules Mcl-1, XIAP, and β-catenin, which are particularly important in the survival of myeloma cells. Omacetaxine mepesuccinate is also known as homoharringtonine, an alkaloid originally discovered and structurally identified from Cephalotaxus harringtonia, which occurs naturally in Japan and eastern Asia.разработк И противоопухолевых препаратов
This compound is isolated from Cephalotaxus harringtonia. A racemic mixture ofharringtonine and homoharringtonine is used for acute and chronic myelogenousleukemia (Shoeb 2006).Homoharringtonine поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-371-66670886 | China | 18754 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21636 | 55 | |
| 18017610038 | CHINA | 3619 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29885 | 58 | |
| +8618080483897 | China | 3772 | 58 | |
| 18905173768 | CHINA | 2972 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +8613259417953 | China | 3000 | 58 | |
| +8613815430202 | CHINA | 376 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 |