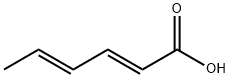
Сорбиновая кислота
- английское имяSorbic acid
- CAS №110-44-1
- CBNumberCB1748891
- ФормулаC6H8O2
- мольный вес112.13
- EINECS203-768-7
- номер MDLMFCD00002703
- файл Mol110-44-1.mol
| Температура плавления | 132-135 °C (lit.) |
| Температура кипения | 228°C |
| плотность | 1.2 g/cm3 at 20 °C |
| давление пара | 0.01 mm Hg ( 20 °C) |
| показатель преломления | 1.4600 (estimate) |
| FEMA | 3921 | 2,4-HEXADIENOIC ACID, (E,E)- |
| Fp | 127 °C |
| температура хранения | 2-8°C |
| растворимость | ethanol: 0.1 g/mL, clear |
| форма | Crystalline Powder |
| пка | 4.76(at 25℃) |
| цвет | White or cream-white |
| Запах | bland |
| РН | 3.3 (1.6g/l, H2O, 20°C) |
| Биологические источники | synthetic |
| Растворимость в воде | 1.6 g/L (20 ºC) |
| Мерк | 14,8721 |
| Номер JECFA | 1176 |
| БРН | 1741831 |
| Стабильность | Material saturated with this acid may ignite spontaneously. Incompatible with strong oxidizing agents. May be light sensitive. |
| ИнЧИКей | WSWCOQWTEOXDQX-MQQKCMAXSA-N |
| LogP | 1.32 at 20℃ |
| FDA 21 CFR | 182.3089; 582.3089; 181.23 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SORBIC ACID |
| Специальный комитет по веществам GRAS | Sorbic acid |
| Справочник по базе данных CAS | 110-44-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | X045WJ989B |
| Справочник по химии NIST | 2,4-Hexadienoic acid, (E,E)-(110-44-1) |
| Система регистрации веществ EPA | Sorbic acid (110-44-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.25 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38-36/38 | |||||||||
| Заявления о безопасности | 26-36-24/25 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | WG2100000 | |||||||||
| F | 8 | |||||||||
| Температура самовоспламенения | >130 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29161930 | |||||||||
| Банк данных об опасных веществах | 110-44-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 7.36 g/kg (Smyth, Carpenter) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P332+P313:При возникновении раздражения кожи: обратиться за медицинской помощью.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
Сорбиновая кислота химические свойства, назначение, производство
Химические свойства
White, crystalline solid. Slightly soluble in water and many organic solvents. Combustible.История
Sorbic acid is a white crystalline solid first isolated in 1859 by hydrolysis of the oil distilled from unripened mountain-ash berries. The name is derived from the scientific term for the rowan tree, Sorbus aucuparia Linne, which is the parent plant of the mountain ash. Sorbic acid was first synthesized in 1900. Interest in this compound was minimal until independent researchers, E. Mueller of Germany and C.M. Gooding of the United States, discovered its antimicrobial effect in 1939 and 1940, respectively. Early interest in manufacturing sorbic acid centered around its use as a tung oil replacement when tung oil supplies were curtailed in the United States during World War II. High manufacturing costs prohibited expanded use until its approval as a food preservative in 1953. Sorbic acid is widely used in foods having a pH of 6.5 or below, where control of bacteria, molds, and yeasts is essential for obtaining safe and economical storage life.Использование
Sorbic Acid is an naturally occurring organic compound first isolated from unripe berries. Sorbic acid has been used as a food preservative and as an inhibitor of Clostridium Botulinum bacteria in mea t products in order to reduce the amount of nitrites which produce carcinogenic nitroamines.Определение
ChEBI: A sorbic acid having trans-double bonds at positions 2 and 4; a food preservative that can induce cutaneous vasodilation and stinging upon topical application to humans. It is the most thermodynamically stable of the four possible geometri isomers possible, as well as the one with the highest antimicrobial activity.Реакции
The chemical reactivity of sorbic acid is determined by the conjugated double bonds and the carboxyl group.Sorbic acid is brominated faster than other olefinic acids. Reaction with hydrogen chloride gives predominately 5-chloro-3-hexenoic acid. Reactions with amines at high temperatures under pressure lead to mixtures of dehydro-2-piperidinones. A yellow crystalline complex is formed from sorbic acid and iron tricarbonyl. Similar coordination occurs also in the presence of other di- and trivalent metals. Reduction of the double bonds can produce various hexenoic acid mixtures.
Общее описание
White powder or crystals. Melting point 134.5°C. Slightly acidic and astringent taste with a faint odor.Реакции воздуха и воды
Soluble in hot water [Handbook of Chemistry and Physics]. May be sensitive to exposure to air and heat. The dust may become explosive, particularly when mixed with free-radical initiators or oxidizing agents. .Профиль реактивности
Sorbic acid may discolor on exposure to light. Can react with oxidizing agents. Also incompatible with bases and reducing agents. The dust may become explosive, particularly when mixed with free-radical initiators or oxidizing agents .Пожароопасность
Sorbic acid is combustible.Профиль безопасности
Moderately toxic by intraperitoneal and subcutaneous routes. Mildly toxic by ingestion. Experimental reproductive effects. A severe human and experimental skin irritant. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use water. When heated to decomposition it emits acrid smoke and irritating fumes.Канцерогенность
Wistar rats (six males) given subcutaneous injections of 2mg sorbic acid, in 0.5mL of arachis oil twice weekly for 65 weeks developed local sarcomas . The first tumor was observed at 82 weeks. Similar findings were also observed in follow-up studies . However, six Wistar rats maintained on drinking water containing 10 mg of sorbic acid/100mL drinking water for 64 weeks did not develop tumors. Tumors also were not observed inWistar rats (50 of each sex) on diets that contained 40 mg/kg/day of sorbic acid for 18 months or in 25 male and female cross-bred white mice after administration of 40 mg/kg/day for 17 months.Mice fed a diet containing 15% sorbic acid for 88 weeks exhibited a high incidence of hepatoma. Furthermore, the glutathione level in the livers of the mice that ingested 15% sorbic acid decreased to 40% of the amount found in controls after a 3-month feeding period; this low level was maintained until the end of the experiments at 12 months. There was a close correlation between the extent of depletion of the glutathione level in the liver and the concentration of sorbic acid added to the diet. In the same strain of mice fed a diet containing 15% sorbic acid for up to 6 months, the acidic fraction of an ether extract showed slight mutagenic activity in an Ames test with Salmonella typhimurium TA98 in the presence of a liver 9000-g supernatant. Consequently, the hepatomas that developed in mice fed a 15% sorbic acid diet were considered to be induced both by the chronic depletion of the hepatic glutathione and by the gradual production of various promutagens in the intestine, which were absorbed and metabolically activated by the liver.
Методы очистки
Crystallise the acid from water. Dry it air or in a desiccator over P2O5. [Beilstein 2 IV 1701.]Сорбиновая кислота запасные части и сырье
сырьё
1of4
запасной предмет
1of2
Сорбиновая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-86-4001020630 +8619831957301 |
China | 1000 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +undefined18602966907 | China | 997 | 58 | |
| +8613031735486 | China | 105 | 58 | |
| +86-15271838296; +8615271838296 |
China | 1512 | 58 | |
| +8619931165850 | China | 1000 | 58 |