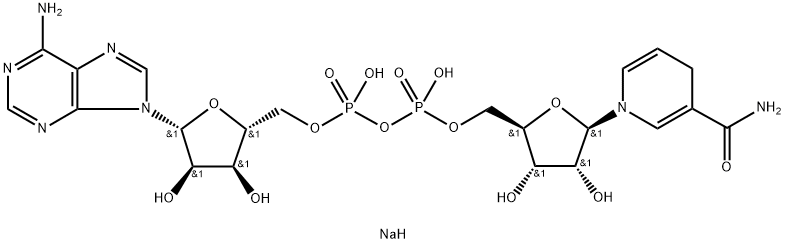
^ Б никотинамид-аденин-динуклеотид уменьшается динатриевой сол
- английское имяNADH, disodium salt
- CAS №606-68-8
- CBNumberCB1709159
- ФормулаC21H30N7NaO14P2
- мольный вес689.44
- EINECS210-123-3
- номер MDLMFCD00036200
- файл Mol606-68-8.mol
химическое свойство
| Температура плавления | 140-142°C |
| плотность | 1.955 at 20℃ |
| давление пара | 0.73Pa at 20-50℃ |
| температура хранения | Inert atmosphere,Store in freezer, under -20°C |
| растворимость | H2O: 50 mg/mL, clear to nearly clear, yellow |
| форма | Powder |
| цвет | Yellow |
| РН | 7.5 (100mg/mL in water, ±0.5) |
| Растворимость в воде | soluble |
| λмакс | 260 and 340 nm |
| БРН | 5230241 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | QRGNQKGQENGQSE-WUEGHLCSSA-L |
| LogP | -1.96 at 21℃ and pH7 |
| Surface tension | 69.22mN/m at 1.022g/L and 20℃ |
| Справочник по базе данных CAS | 606-68-8 |
| FDA UNII | 8295030YNC |
| Система регистрации веществ EPA | Reduced .beta.-nicotinamide adenine dinucleotide disodium salt (606-68-8) |
| UNSPSC Code | 41106305 |
| NACRES | NB.21 |
больше
| Коды опасности | Xn |
| Заявления о рисках | 23/24/25-36/37/38-39/23/24/25-40-22 |
| Заявления о безопасности | 26-36/37/39-45-24/25-36/37 |
| WGK Германия | 3 |
| F | 3-10-23 |
| TSCA | Yes |
| кода HS | 29349990 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
^ Б никотинамид-аденин-динуклеотид уменьшается динатриевой сол химические свойства, назначение, производство
Описание
β-Nicotinamide adenine dinucleotide (NAD+) and β-Nicotinamide adenine dinucleotide, reduced (NADH) comprise a coenzyme redox pair (NAD+:NADH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. In addition to its redox function, NAD+/NADH is a donor of ADP-ribose units in ADP-ribosylaton (ADP-ribosyltransferases; poly(ADP-ribose) polymerases ) reactions and a precursor of cyclic ADP-ribose (ADP-ribosyl cyclases).Химические свойства
White to beige powderОпределение
NADH, disodium salt is a dehydrogenase complex that is the reduced formof NAD. As a reagent, NADH can be used in enzyme cycling assays to amplify detection of activity of biologically relevant enzymes or metabolites present in low concentrations.Биологические функции
NADH disodium salt (Disodium NADH) is an orally active reduced coenzyme. NADH disodium salt is a donor of ADP-ribose units in ADP-ribosylaton reactions and a precursor of cyclic ADP-ribose. NADH disodium salt plays a role as a regenerative electron donor in cellular energy metabolism, including glycolysis, β-oxidation and the tricarboxylic acid (TCA) cycle.Общее описание
β-Nicotinamide adenine dinucleotide (β-NAD) regulates energy metabolism and immunity. It is a cofactor for mitochondrial deacetylase sirtuin-3 enzyme and modulates inflammasome assembly. β-NAD supresses interleukin-1β levels in monocytic cells in inflammatory syndromes. β-NAD released by neurosecretory cells is a potential neurotransmitter. β-NAD is a vascular mediator in lung endothelial cells and may play a protective role against cytokine mediated inflammation.Биохимия/физиол Действия
NADH is a coenzyme that functions as a regenerating electron donor in catabolic processes including glycolysis, β-oxidation and the citric acid cycle (Krebs cycle, TCA cycle). It participates in cell signaling events as well, for example as a substrate for the poly (ADP-ribose) polymerases (PARPs) during the DNA damage response. The NAD+/NADH dependent sirtuins play key roles in stress responses during events involving energy metabolism, with implications in cancer biology, diabetes and neurodegenerative disease.Приложение биотехнологий?
Reduced β-nicotinamide adenine dinucleotide (NADH) plays a major role in metabolism as a cofactor in redox reactions and as a mobile electron carrier. NADH is a high energy compound that donates electrons to the electron transport chain to provide energy for ATP production by oxidative phosphorylation. NADH is a required oxidizing cosubstrate in fermentation, which regenerates NAD. NADH is fluorescent, which provides for a relatively simple way to detect NADH in biological samples. NADH is also used in enzyme cycling assays to detect relevant biological molecules in tissues.в пробирке
NADH is unstable under acidic conditions but it is stable under alkaline conditions.NADH (0-1 mM; 0-12 h) increases NAD levels in various mammalian cell lines+.
NADH (1 mM; 24 h) causes low toxicity and protects cells from genotoxicity.
Методы очистки
This coenzyme is available in high purity, and it is advisable to buy a fresh preparation rather than to purify an old sample as purification will invariably lead to a more impure sample contaminated with the oxidised form (NAD). It has UV max at 340nm ( 6,200 M-1cm-1) at which wavelength the oxidised form NAD has no absorption. At 340nm a 0.161mM solution in a 1cm (pathlength) cell has an absorbance of 1.0 unit. The purity is best checked by the ratio A280nm/A340nm ~2.1, a value which increases as oxidation proceeds. The dry powder is stable indefinitely at -20o. Solutions in aqueous buffers at pH ~7 are stable for extended periods at -20o and for at least 8hours at 0o, but are oxidised more rapidly at 4o in a cold room (e.g. almost completely oxidised overnight at 4o). [UV: Drabkin J Biol Chem 175 563 1945, Fluorescence: Boyer & Thorell Acta Chem Scand 10 447 1956, Redox: Rodkey J Biol Chem 234 188 1959, Schlenk in The Enzymes 2 250, 268 1951, Kaplan in The Enzymes 3 105, 112 1960.] Deuterated NADH, i.e. NADD, has been purified through the anion exchange resin AG-1 x 8 (100-200 mesh, formate form) and through a Bio-Gel P-2 column. [Viola et al. Anal Biochem 96 334 1979, Beilstein 26 III/IV 3639.]Определение
The NAD+ Is the oxidized form, that is, a state in which it loses an electron. NADH is a reduced form of the molecule, which means that it gains the electron lost by NAD+. Redox reactions involving electron transfers play a central role in energy creation. When NAD+ takes an electron from glucose, it becomes NADH. NADH transports this electron to mitochondria where the cell can take the energy that is stored in the electron. NADH then donates the electron to oxygen, converting it back to NAD+.^ Б никотинамид-аденин-динуклеотид уменьшается динатриевой сол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-75589664337 +86-15112661142 |
China | 58 | 58 | ||
| 029-86107037 13289246953 |
China | 1169 | 58 | ||
| +86-13545906766; +8613545906766 |
China | 263 | 58 | ||
| +86-021-68187180-811 +86-13681683526 |
China | 188 | 58 | ||
| +86-86-+86-86-029-87551862 +8617702909819 |
China | 2608 | 58 | ||
| +8618058761490 | China | 49983 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | ||
| +8615669938129 | China | 130 | 58 | ||
| +86-15373193816 +86-15373193816 |
China | 269 | 58 | ||
| +86-86-18382622232 +8618382622232 |
China | 9 | 58 |
^ Б никотинамид-аденин-динуклеотид уменьшается динатриевой сол Обзор)
- ^ Б-Никотинамидадениндинуклеотид
- 6-Тиогуанин
- Коэнзим а
- Никотинамид
- Sodium phosphate dibasic dodecahydrate BioXtra
- Убидекаренон
- Этилендиаминтетрауксусной кислоты динатриевая сол
- Acetyl coenzyme?A sodium salt
- Аденин
- EDTA динатриевая соль дигидрат EP
1of4