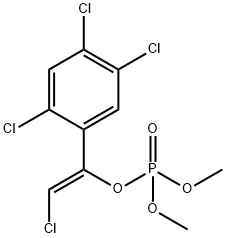
Tetrachlorvinphos
- русский язык имя
- английское имяTetrachlorvinphos
- CAS №22248-79-9
- CBNumberCB1673649
- ФормулаC10H9Cl4O4P
- мольный вес365.96
- EINECS244-865-4
- номер MDLMFCD00078729
- файл Mol22248-79-9.mol
химическое свойство
| Температура плавления | 97-98 °C(lit.) |
| Температура кипения | 399.5±42.0 °C(Predicted) |
| плотность | 1.520±0.06 g/cm3(Predicted) |
| давление пара | 6.0×10-6 Pa (20 °C) |
| температура хранения | 0-6°C |
| растворимость | Acetonitrile (Slightly), Methanol (Slightly) |
| форма | solid |
| Растворимость в воде | 11mg l-1(20°C) |
| Мерк | 13,9267 |
| Справочник по базе данных CAS | 22248-79-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | DM33MW89QU |
| Предложение 65 Список | Tetrachlorvinphos |
| МАИР | 2B (Vol. 30, Sup 7, 112) 2017 |
| Справочник по химии NIST | Tetrachlorvinphos(22248-79-9) |
| Система регистрации веществ EPA | Tetrachlorvinphos (22248-79-9) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,N,F |
| Заявления о рисках | 21/22-50/53-36-20/21/22-11-22 |
| Заявления о безопасности | 36-60-61-36/37-16 |
| РИДАДР | UN 3077 9/PG 3 |
| RTECS | TB9100000 |
| кода HS | 29199000 |
| Банк данных об опасных веществах | 22248-79-9(Hazardous Substances Data) |
| Токсичность | LD50 in male, female rats (mg/kg): 1100, 1125 orally (Gaines) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Tetrachlorvinphos химические свойства, назначение, производство
Описание
Tetrachlorvinphos was initially registered for use in the United States in 1966 for use on various food crops, livestock, and pet animals, and in around buildings. Its use on food crops were voluntarily canceled in the United States in 1987; however, it is used on food crops in developing countries. Tetrachlorvinphos is sold under the trade names Rabon and Gardona.Химические свойства
Technical tetrachlorvinphos is a tan-to-brown crystalline solid. Tetrachlorvinphos is stable at ,100 C and slowly hydrolyzed at 50°C. Aromatic odor.Soluble in water at 24°C 15 ppm; limited solubility in most aromatic hydrocarbons.
Использование
Tetrachlorvinphos is used to control lepidopterous and dipterous larvae in fruit and lepidopterous larvae in cotton, maize, rice, tobacco and vegetables. It is also used against nuisance flies in animal houses, animal ectoparasites and stored product pests.Опасность
Cholinesterase inhibitor. Questionable carcinogen.Канцерогенность
When rats were given diets with 0, 4250, or 8500 ppm tetrachlorvinphos for 80 weeks, both males and females had a high incidence of thyroid C-cell hyperplasia, and females had increased incidences of adrenal cortical adenomas and thyroid C-cell adenomas .Экологическая судьба
Tetrachlorvinphos is nonpersistent in the environment. The primary route of dissipation is through biotic degradation. Based on its use pattern, risks of contamination of groundwater or surface water by tetrachlorvinphos are minimal.Метаболический путь
The chemical structure of tetrachlorvinphos is very close to that of chlorfenvinphos and the routes of metabolic breakdown have been shown to be very similar. Technical tetrachlorvinphos is usually >95% Z-isomer, unlike chlorfenvinphos which is an E/Z mixture. As with chlorfenvinphos, the major routes of detoxification are by dealkylation and hydrolysis to yield desmethyltetrachlorvinphos and 2,2’,4’,5’- tetrachloroacetophenone plus dimethyl phosphate, respectively. Further metabolism of the chloroacetophenone moiety then leads, via reduction or hydrolysis and glutathione-dependent displacement of the side chain chlorine substituent, to the formation of 1-(2,4,5-trichlophenyl) ethane-l,Z-diol and 1-(2,4,5-trichlorophenyl)ethan-l-owl hich are conjugated with glucose or glucuronic acid to afford the ultimate metabolites. Oxidation of the β carbon atom to give 2,4,5-trichloromandelic acid followed by decarboxylation leads to the formation of 2,4,5-trichlorobenzoic acid which is conjugated with glycine in some mammals as the final metabolite. The metabolic routes were summarised by Beynon et al. (1973).Tetrachlorvinphos запасные части и сырье
сырьё
1of4
запасной предмет
Tetrachlorvinphos поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +86-85511178; +86-85511178; |
China | 35425 | 58 |