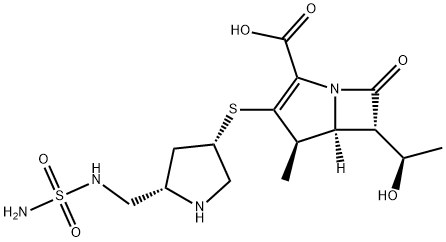
Дорипенем
- английское имяDoripenem
- CAS №148016-81-3
- CBNumberCB1547391
- ФормулаC15H24N4O6S2
- мольный вес420.5
- EINECS1308068-626-2
- номер MDLMFCD02092739
- файл Mol148016-81-3.mol
химическое свойство
| Температура плавления | >186°C dec. |
| Температура кипения | 694.8±65.0 °C(Predicted) |
| плотность | 1.59±0.1 g/cm3(Predicted) |
| температура хранения | Sealed in dry,Store in freezer, under -20°C |
| растворимость | In water, not known, in DMSO 20 mg/ml, in pBS 3 mg/ml |
| форма | Solid |
| пка | 4.27±0.60(Predicted) |
| цвет | White to Light Beige |
| Стабильность | Hygroscopic |
| Справочник по базе данных CAS | 148016-81-3 |
| FDA UNII | BHV525JOBH |
| Код УВД | J01DH04 |
Дорипенем химические свойства, назначение, производство
Описание
Doripenem monohydrate is an ultra-broad-spectrum injectable β-lactam antibiotic and belongs to the subgroup of carbapenems. It was introduced by Shionogi Co. of Japan under the brand name Finibax in 2005 and is being marketed outside Japan by Johnson & Johnson. Doripenem act by decreases the process of cell wall growth, which eventually leads to elimination of the infectious cell bacteria together. It is used for treatment of bacterial respiratory and urinary tract infections. Doripenem is a 1β-methyl carbapenem derivative, and it is the fourth analog to be marketed in this series following the launch of meropenem, biapenem, and ertapenem in previous years. The introduction of a 1β-methyl group to the carbapenem skeleton enhances metabolic stability to renal dehydropeptidase-1 (DHP-1) and leads to improved antibacterial potency.Химические свойства
Doripenem white to somewhat yellowish crystalline powder which is moderately soluble in water, slightly soluble in methanol, and virtually insoluble in ethanol. Doripenem is also solution in N,N-dimethylformamide. The chemical configuration of doripenem’s has 6 asymmetrical carbon atoms (6 stereocenters) and is most commonly supplied as one pure isomer. In terms of doripenem for injection, the crystallized powered drug can form a monohydrate when mixed with water.Использование
Doripenem hydrate is used to treat complicated urinary infection including Pyelonephritis caused by E.coli. Doripenem hydrate is promoted in the United States as DORIBAX(R). This drug is synthesized from p-nitrobenzyl-protected enolphosphate 2b and N-(p-nitrobenzyloxycarbonyl)-protected aminomethylpyrrolidine.Имя бренда
Doribax,FinibaxАнтимикробная активность
Doripenem have a broad spectrum of bacterial activity including both gram-positive and gram-negative bacteria but it is not active against MRSA. It is stable against β-lactamases including those with extended spectrum, but it is susceptible to the action of carbapenemases (Mandell, 2009). Thus it can be used in the treatment of infections such as: complex abdominal infections, pneumonia within the setting of a hospital, and complicated infections of the urinary tract including kidney infections with septicemia. Doripenem is also more active against Pseudomonas aeruginosa then other carbapenems.Фармакокине?тика
Cmax 500 mg intravenous infusion (1 h): c. 23 mg/L after 1 h500 mg intravenous infusion (4 h): c. 8 mg/L
Plasma half-life: 1 h
Volume of distribution: 16.8 L (steady state)
Plasma protein binding: 8.1%
Absorption and distribution
Doripenem is not absorbed after oral administration. It penetrates well into most tissues and fluid, achieving concentrations matching or exceeding those required to inhibit most susceptible bacteria at the site of infection for the approved indications.
Metabolism and excretion
Metabolism of doripenem to the microbiologically inactive ring-opened metabolite occurs primarily by renal dehydropeptidase. Based on area under the concentration–time curve (AUC) values in plasma following a single 500 mg dose in healthy volunteers, 18% appears as metabolite and the rest as unchanged drug.
Excretion is primarily by the renal route. Within 24 h after dosing, 78.7% and 18.5% of the dose was recovered in urine as unchanged drug and the ring-opened metabolite, respectively. After administration of radiolabeled doripenem, 0.7% of the total radioactivity was recovered in feces after 1 week.
Клиническое использование
Doripenem is indicated for use for the treatment of intra-abdominal infections, and complicated urinary tract infections.Complicated urinary tract infections, including pyelonephritis
Nosocomial pneumonia, including ventilator-associated pneumonia (Europe)
Побочные эффекты
The most commonly reported adverse effects of doripenem include pain or swelling at the injection site, nausea, headache, and diarrhea.Seizure and central nervous system (CNS) side effects are observed rarely (<1%), though headache is reported by 2.3% of patients. Other common drug-related adverse reactions are diarrhea (2.0%), nausea (1.9%), anemia (1.4%) and phlebitis (1.4%). Hypersensitivity reactions related to intravenous administration of the study drug and Clostridium difficile colitis occurred at a rate of less than 1%. However, patients with a history of hypersensitivity reactions to other β-lactam agents should be treated cautiously.
Способ действия
Doripenem's bactericidal function is due to its inhibition of the third stage of bacterial cell wall synthesis. Binding to penicillin-binding proteins weakens the cell wall and leads to cell death due to lysis of the cell wall.Doripenem is effective against both grampositive and gramnegative aerobic bacteria.It may be more potent in vitro against Pseudomonas aeruginosa than meropenem.
Дорипенем запасные части и сырье
Дорипенем поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8617732866630 | China | 8773 | 58 | |
| +8613288715578 | China | 12303 | 58 | |
| +undefined18602966907 | China | 997 | 58 | |
| +8618092446649 | China | 1143 | 58 | |
| +86-17320513646 +8617320513646 |
China | 10000 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21622 | 55 | |
| +86-21-33585366 - 03@ | CHINA | 738 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29855 | 58 | |
| 0086-13720134139 | CHINA | 965 | 58 | |
| 21-33585366 | CHINA | 1319 | 58 |