Ферроцен
- английское имяFerrocene
- CAS №102-54-5
- CBNumberCB1414721
- ФормулаC10H10Fe
- мольный вес186.03
- EINECS203-039-3
- номер MDLMFCD00001427
- файл Mol102-54-5.mol
| Температура плавления | 172-174 °C (lit.) |
| Температура кипения | 249 °C (lit.) |
| плотность | 1.490 |
| Плотность накопления | 500kg/m3 |
| давление пара | 0.03 mm Hg ( 40 °C) |
| Fp | 100°C |
| температура хранения | Store below +30°C. |
| растворимость | insoluble in H2O; soluble in ethanol, ethyl ether,benzene, dilute HNO 3 |
| форма | crystal |
| цвет | orange |
| Растворимость в воде | practically insoluble |
| Чувствительный | Air & Moisture Sensitive |
| λмакс | 358 nm |
| Сублимация | 100 ºC |
| Мерк | 14,4037 |
| Пределы воздействия | ACGIH: TWA 10 mg/m3; TWA 1 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 10 mg/m3; TWA 5 mg/m3; TWA 1 mg/m3 |
| Стабильность | Stable at room temperature. Incompatible with strong oxidizing agents. Highly flammable. |
| LogP | 3.711 at 22℃ |
| Справочник по базе данных CAS | 102-54-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | U96PKG90JQ |
| Справочник по химии NIST | Ferrocene(102-54-5) |
| Система регистрации веществ EPA | Ferrocene (102-54-5) |
| UNSPSC Code | 12352103 |
| NACRES | NA.22 |
| Коды опасности | F,Xn,N | |||||||||
| Заявления о рисках | 11-22-51/53-2017/11/22 | |||||||||
| Заявления о безопасности | 61-22-24/25 | |||||||||
| РИДАДР | UN 1325 4.1/PG 2 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 10 mg/m3 (total) | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | LK0700000 | |||||||||
| Температура самовоспламенения | >150 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29310095 | |||||||||
| Банк данных об опасных веществах | 102-54-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 1320 mg/kg LD50 dermal Rat > 3000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H302+H332:Вредно при проглатывании или при вдыхании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H228:Воспламеняющееся твердое вещество.
H360FD:Может отрицательно повлиять на способность к деторождению. Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Ферроцен химические свойства, назначение, производство
Химические свойства
Ferrocene, a metallocene, is a bright orange salt-like crystals from alcohol. Camphor odor.Физические свойства
Orange crystals; camphor-like odor; melts at 172.5°C; vaporizes at 249°C; sublimes above 100°C; thermally stable above 500°C; insoluble in water; soluble in alcohol, ether and benzene; also soluble in dilute nitric acid and concentrated sulfuric acid forming a deep red solution that fluoresces.Использование
Ferrocene is used as a catalyst for vulcanization, acceleration, and polymerization, as a chemical intermediate for polymeric compounds such as high temperature polymers, as an antiknock additive for gasoline, as a coating for missiles and satellites, and as a high-temperature lubricant.Методы производства
Ferrocene is produced from the reaction of cyclopentadiene with reduced iron in the presence of metal oxides. There is also a two-stage production process in which produced iron (II)oxide (stage 1) is reacted with cyclopentadiene.Определение
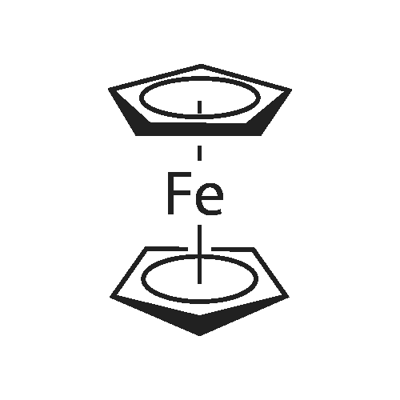
ferrocene: An orange-red crystallinesolid, Fe(C5H5)2; m.p. 173°C. Itcan be made by adding the ioniccompound Na+C5H5- (cyclopentadienylsodium, made from sodium andcyclopentadiene) to iron(III) chloride.In ferrocene, the two rings are parallel,with the iron ion sandwiched betweenthem (hence the namesandwich compound: see formula).The bonding is between pi orbitalson the rings and d-orbitals on theFe2+ ion. The compound can undergoelectrophilic substitution on theC5H5rings (they have some aromatic character).It can also be oxidized to theblue ion (C5H5)2Fe+. Ferrocene is the first of a class of similar complexescalled sandwich compounds. Its systematicname is di-π-cyclopentadienyliron(II).Подготовка
Dicyclopentadienyliron may be obtained in a single-step synthetic route by heating cyclopentadiene with iron or iron pentacarbonyl at 300°C:2C5H5 + Fe → (C5H5)2Fe
Also, it can be prepared by the reaction of iron(II) chloride with cyclopentadiene in the presence of an alkyl amine or a similar base.
Another convenient method of preparing this π-complex of iron is a twostep process in which the first step involves preparation of cyclopentadienyl Grignard reagent, such as 2,4-cyclopentadienylmagnesium bromide C5H5MgBr which may then be combined with ferric chloride to yield dicyclopentadienyl iron:
3C5H5MgBr + FeCl3 → (C5H5)2Fe + 3MgBrCl
Another general method of preparation involves the reaction of cyclopentadiene with sodium metal or sodium hydride in tetrahydrofuran (THF). Addition of iron(II) chloride to this solution forms the complex dicyclopentadienyliron:
2C5H6 + 2Na → 2C5H5ˉ + 2Na+ + H2
In 3:2 molar ratio of cyclopentadiene to sodium cyclopentene is obtained along with cyclopentadienidide (C5H5ˉ ) anion:
3C5H6 + 2Na → 2C5H5¯ + 2Na+ + C5H8
FeCl2 + 2C5H6Na → (C5H5)2Fe + 2NaCl
Общее описание
Orange crystalline solid or orange-yellow powder. Sublimes above 212°F. Camphor odor.Реакции воздуха и воды
Sensitive to prolonged exposure to air and may be sensitive to light. Insoluble in water.Профиль реактивности
Ferrocene reacts violently with tetranitromethane. . Contact of tetranitromethane with Ferrocene under various conditions leads to violent explosion, [Trans. Met. Chem., 1979, 4, 207-208].Опасность
Moderate fire risk. Evolves toxic products on decomposition and heating.Угроза здоровью
Dicyclopentadienyl iron causes changes in blood parameters and hepatic cirrhosis.The toxicological properties of dicyclopentadienyl iron have not been extensively investigated. However, it has been used as a preventive and therapeutic iron deficiency drug, and its utilization is listed as tolerable.
Пожароопасность
Flash point data for Ferrocene are not available. Ferrocene is probably combustible.Профиль безопасности
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Flammable; reacts violently with NH4ClO4. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
Used as additive in fuel oil; antiknock agent in gasoline fuel; used in making rubber, silicone resins, high-temperature polymers and lubricants; interme diate for high-temperature polymers; as a smoke suppres sant and catalystКанцерогенность
Ferrocene was administered by intramuscular injection at a dose of 5175 mg/kg/2 years. By the criterion established by the Registry of Toxic Effects of Chemical Substances (RTECS), ferrocene was an equivocal tumorigenic agent and tumors were most evident at the site of multiple injections.Перевозки
UN1325 Flammable solids, organic, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid.Методы очистки
Purify it by crystallisation from pentane or cyclohexane (also *C6H6 or MeOH can be used). It is moderately soluble in Et2O and sublimes readily above 100o. Crystallisation from EtOH gave material m 172.5-173o. [Wilkinson Org Synth Coll Vol IV 473 1963, Miller J Chem Soc 632 1952.] It has also been crystallised from methanol and sublimed in vacuo. [Saltiel et al. J Am Chem Soc 109 1209 1987, Beilstein 16 IV 1783.]Несовместимости
Violent reaction with ammonium per chlorate, tetranitromethane, mercury(II) nitrate. Incompa tible with oxidizers (chlorates, nitrates, peroxides, perman ganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.Peroxomonosulfuric acid. Decomposes @≧465 ℃.
Ферроцен запасные части и сырье
сырьё
1of2
запасной предмет
- 1,1'-бис(ди-трет-бутилфосфино)ферроцен палладий дихлорид в компании Реарус
- 1,1 '-Ferrocenedicarboxaldehyde
- 1 1'-BIS(DIPHENYLPHOSPHINO)FERROCENE
- 1,1'-бис(дифенилфосфино)ферроцен
- 1,1'-DI-N-BUTYLFERROCENE
- 1,1'-бис(ди-трет-бутилфосфино)ферроцен
- Ferrocene derivative
- dibuty cebacate with ferric chloride
- н-Butylferrocene
- 1,1 '-Dimethylferrocene
1of4
Ферроцен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +undefined18525718838 | China | 1 | 58 | ||
| +8613256193735 | China | 988 | 58 | ||
| 0411-84820922 8613904096939 |
China | 304 | 57 | ||
| +86-13806087780 | China | 17365 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | ||
| +undefined-86-1906231316-9 +undefined86-19062313169 |
China | 712 | 58 | ||
| China | 5701 | 58 | |||
| +8615350571055 | China | 6087 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 |