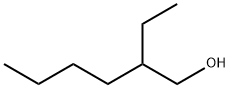
2-Этилгексанол
- английское имя2-Ethylhexanol
- CAS №104-76-7
- CBNumberCB1394458
- ФормулаC8H18O
- мольный вес130.23
- EINECS203-234-3
- номер MDLMFCD00004746
- файл Mol104-76-7.mol
| Температура плавления | −76 °C(lit.) |
| Температура кипения | 183-186 °C(lit.) |
| плотность | 0.833 g/mL at 25 °C(lit.) |
| плотность пара | 4.49 (vs air) |
| давление пара | 0.2 mm Hg ( 20 °C) |
| показатель преломления | n |
| FEMA | 3151 | 2-ETHYL-1-HEXANOL |
| Fp | 171 °F |
| температура хранения | Store below +30°C. |
| растворимость | Water |
| пка | 15.05±0.10(Predicted) |
| форма | Liquid |
| цвет | Clear |
| Запах | sweet. |
| РН | 7 (1g/l, H2O, 20℃) |
| Пределы взрываемости | 0.88%, 104°F |
| Порог?обнаружения?запаха? | 0.013ppm |
| Odor Type | citrus |
| Скорость испарения | 0.01 |
| Биологические источники | synthetic |
| Растворимость в воде | 1 g/L (20 ºC) |
| Номер JECFA | 267 |
| Мерк | 14,3808 |
| БРН | 1719280 |
| Диэлектрическая постоянная | 3.4(18℃) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids. |
| ИнЧИКей | YIWUKEYIRIRTPP-UHFFFAOYSA-N |
| LogP | 2.9 at 25℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | 2-ETHYL-1-HEXANOL |
| FDA 21 CFR | 175.105; 175.300 |
| Справочник по базе данных CAS | 104-76-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | XZV7TAA77P |
| Справочник по химии NIST | 1-Hexanol, 2-ethyl-(104-76-7) |
| Система регистрации веществ EPA | 2-Ethylhexanol (104-76-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 37/38-41-36-21-52/53-36/37/38-20 | |||||||||
| Заявления о безопасности | 26-36/39-36/37/39-36-61 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | MP0350000 | |||||||||
| Температура самовоспламенения | 550 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29051610 | |||||||||
| кода HS | 29339990 | |||||||||
| Банк данных об опасных веществах | 104-76-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 3730 mg/kg LD50 dermal Rat > 3000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
2-Этилгексанол химические свойства, назначение, производство
Описание
2-Ethylhexanol, also known as 2-Ethylhexan-1-ol, abbreviated as 2-EH, is a branched eight-carbon alcohol. In industry, 2-EH is mainly used to make ester plasticizers, which can be used to produce soft polyvinyl chloride. 2-EH can also be used to make a chemical used in the production of coating materials, adhesives, printing inks and impregnation agents. 2-EH occurs naturally in food and is used as a volatile flavoring agent and is indirectly approved as a food additive by the FDA. In addition, 2-EH is also used as an inert pesticide ingredient in pesticide formulations for cultivated crops, raw agricultural products (RAG) or animals.Химические свойства
2-Ethylhexanol is a clear, colorless to pale yellow oily liquid. It has a mild, oily, sweet, slightly floral odor reminiscent of rose and sweet, fatty-floral taste with a fruity note. Soluble in 720 times water, miscible in most organic solvents.Вхождение
Reported found in papaya, peach, pear, blackberry, strawberry, cabbage, Parmesan and mozzarella cheese, butter, roasted chicken, cognac, sherry, grape wines, tea, avocado, kiwifruit, crab and clam.Использование
2-Ethylhexanol is the most important C8 alcohol and is mainly used as the alcohol component for the manufacture of ester plasticizers for soft poly(vinyl chloride) (PVC). Other minor uses include the manufacturing of 2-ethylhexyl acrylate, as a dispersing agent and wetting agent, as a solvent for gums and resins, as a cosolvent for nitrocellulose, and in ceramics, paper coatings, rubber latex, textiles, and fragrances.Подготовка
2-ethylhexanol synthesis: Condensation of acetaldehyde into butanol aldehyde, dehydration to obtain crotonaldehyde, hydrogenation to n-butyraldehyde, condensation dehydration to obtain 2-ethyl-2-hexenal, and then hydrogenation to obtain 2-ethylhexanol.Определение
ChEBI: 2-Ethylhexanol is a primary alcohol that is hexan-1-ol substituted by an ethyl group at position 2. It has a role as a volatile oil component and a plant metabolite.прикладной
2-Ethyl-1-hexanol is suitable for use in a study to compare its susceptibilities of dynamic heat capacity and dielectric polarization under isothermal conditions. It may be used to study lipase-catalyzed transesterification (alcoholysis) of rapeseed oil and 2-ethyl-1-hexanol in the absence of solvent. 2-Ethyl-1-hexanol may be used in broadband dielectric spectroscopy studies of the polyalcohols-glycerol, xylitol and sorbitol. It may be used in the preparation of porous beads.Общее описание
2-ethyl hexanol appears as a dark brown liquid with an aromatic odor. Insoluble in water and less dense than water. Flash point between 140 - 175°F. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
2-Ethylhexanol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. 2-Ethylhexanol is incompatible with strong oxidizing agents and strong acids.Угроза здоровью
Anesthesia, nausea, headache, dizziness; mildly irritating to skin and eyes.Пожароопасность
2-Ethylhexanol is combustible.Профиль безопасности
Moderately toxic by ingestion, skin contact, intraperitoneal, subcutaneous, and parented routes. An experimental teratogen. Other experimental reproductive effects. A severe eye and moderate skin irritant. Mutation data reported. A dangerous fire hazard when ex posed to heat or flame; can react vigorously with oxidzing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also ALCOHOLS.Канцерогенность
Male and female F344 rats were dosed by oral gavage with 0, 50, 150, or 500 mg/kg 2-ethylhexanol (0.005% in aqueous Cremophor EL, a polyoxyl 35 castor oil), 5 days/week for 2 years. There were no differences of biological importance between the vehicle control and a water control group that was included in the study. There was no evidence of treatment-related neoplastic lesions in any of the exposed groups.2-Этилгексанол запасные части и сырье
сырьё
1of2
запасной предмет
- polyoxyethylene isooctyl ether phosphate
- 2-ЭТИЛГЕКСИЛАКРИЛАТ
- AZELAIC ACID DI(2-ETHYLHEXYL) ESTER
- ISOOCTYL THIOGLYCOLATE
- 2-метил-1-пропанол
- Триизооктилфосфит
- 2-Ethylhexyl diphenyl phosphate
- БИС(2-ЭТИЛГЕКСИЛ)ФОСФАТ
- Диоктил гексадиоат
- Трис(2-этилгексил)фосфат
- Бис(2-этилгексил) себакат
- Dioctyl maleate
- 2-этилгексановой кислоты
- 2-МЕТОКСИ-5- (2'-ЭТИЛГЕКСИЛОКСИ) БЕНЗОЛ-1 и
- SULFONATED ALIPHATIC POLYESTER
- SEBACIC ACID DI-N-OCTYL ESTER
- Ди-(2-этилгексил)4,5-эпокситетрагидрофталат
- 2-этилгексил бромид
- Ди-н-октил фтала
- Ди-(2-этилгексил)пероксидикарбонат
- бис (2-этилгексил) фенилфосфит
- НАТРИЙ диоктилсульфосукцинат
- Бис (2-этилгексил) адипа
- 2-этилгексилгидроген-2-этилгексилфосфонат
- Diisooctyl sebacate
1of7
2-Этилгексанол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613325218432 | China | 57 | 58 | ||
| +86-25-86655873 +8613962173137 |
China | 193 | 55 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +16837774622 | China | 295 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | ||
| +8613343047651 | China | 3692 | 58 |