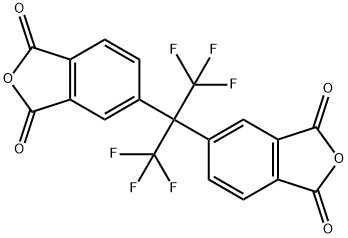
4,4 '- (гексафторизопропилиден) дифталевого ангидрида
- английское имя4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
- CAS №1107-00-2
- CBNumberCB1298806
- ФормулаC19H6F6O6
- мольный вес444.24
- EINECS214-170-0
- номер MDLMFCD00039143
- файл Mol1107-00-2.mol
| Температура плавления | 244-247 °C(lit.) |
| Температура кипения | 494.5±45.0 °C(Predicted) |
| плотность | 1.697±0.06 g/cm3(Predicted) |
| давление пара | 7.7Pa at 20℃ |
| температура хранения | Keep in dark place,Sealed in dry,Room Temperature |
| форма | powder to crystal |
| цвет | White to Almost white |
| Растворимость в воде | Miscible with water. |
| Чувствительный | Moisture Sensitive |
| БРН | 7057916 |
| InChI | InChI=1S/C19H6F6O6/c20-18(21,22)17(19(23,24)25,7-1-3-9-11(5-7)15(28)30-13(9)26)8-2-4-10-12(6-8)16(29)31-14(10)27/h1-6H |
| ИнЧИКей | QHHKLPCQTTWFSS-UHFFFAOYSA-N |
| SMILES | C(C1C=CC2C(=O)OC(=O)C=2C=1)(C1C=CC2C(=O)OC(=O)C=2C=1)(C(F)(F)F)C(F)(F)F |
| LogP | 5.59 at 25℃ |
| Справочник по базе данных CAS | 1107-00-2(CAS DataBase Reference) |
| FDA UNII | N2GA6JCU7H |
| Система регистрации веществ EPA | 1,3-Isobenzofurandione, 5,5'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylidene]bis- (1107-00-2) |
| UNSPSC Code | 12162002 |
| NACRES | NA.23 |
| Коды опасности | C,Xi | |||||||||
| Заявления о рисках | 34 | |||||||||
| Заявления о безопасности | 26-36/37/39-45 | |||||||||
| РИДАДР | UN 3261 8/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| F | 21 | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | T | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29173990 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H335:Может вызывать раздражение верхних дыхательных путей.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
4,4 '- (гексафторизопропилиден) дифталевого ангидрида химические свойства, назначение, производство
Описание
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA) is an aromatic organofluorine compound that is utilized as a dianhydride monomer. It is among the most frequently used dianhydride monomers due to its exceptional balance of mechanical and electrical properties. It is particularly prominent in the construction of colorless and transparent polyimides.Физические свойства
Beige crystalприкладной
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA) can be used:(1) With APAF as dianhydride monomer, it can be used to prepare thermally rearranged polybenzoxazole (TR-PBO) gas separation membranes by thermal rearrangement reaction at 450°C. The product TR-PBO membrane has excellent gas permeation selectivity and high permeability. the H2/CH4 separation performance of APAF-6FDA membrane is close to the Robertson upper limit of 2008 [5].
(2) Used as a raw material for the preparation of thermoplastic fluorinated polyimide resin.[6].
(3) Polymers for electronic materials;
(4) Polyimide materials. These materials can be used as substrates for wearable devices and as multilayer insulation materials in order to protect against space dust, cosmic rays and satellite debris[7].
Общее описание
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA) consists of two phthalic anhydrides bridged with a perfluoroisopropyl group and is mainly used in the synthesis of polyimides. Polyimides from 6FDA are commonly used in hybrid matrix membranes. The polyimide matrix provides good dispersion of the filler and can be loaded with up to 50 wt% filler. These membranes have been used for highly selective gas separation (CO2/CH4) and solar cell encapsulation (power conversion efficiency of silicon-based solar cells increased from 14.67% to 15.64%).преимущество
The 4,4'-(hexafluoroisopropylidene) diphthalic anhydride (6FDA) monomer contains rigid benzene ring groups, and so, the resulting polymer has excellent mechanical properties. 6FDA contained a stable benzene ring and fluorine atoms. Due to the strong electronegativity of the fluorine atoms and the stability of the benzene ring, the produced polycarbonate had better thermal stability[1-2].Меры предосторожности
The storage precautions for 6FDA are as follows: The chemical must be stored in a cool and well-ventilated warehouse, away from sources of heat and fire. Proper sealing is required to prevent contact with air, and it should always be kept separate from oxidants, edible chemicals, and acids. The storage area should have explosion-proof lighting and ventilation facilities and must not allow any mechanical equipment or tools that may cause sparks to prevent accidents. Leakage emergency treatment equipment and suitable storage materials must be readily available in the storage area.использованная литература
[1] Yile Zhang. “Poly(propylene carbonate) networks with excellent properties: Terpolymerization of carbon dioxide, propylene oxide, and 4,4?-(hexafluoroisopropylidene) diphthalic anhydride.” e-Polymers 21 1 (2021): 511–519.[2] C. D. Simone, D. A. Scola. “Phenylethynyl End-Capped Polyimides Derived from 4,4‘-(2,2,2-Trifluoro-1-phenylethylidene)diphthalic Anhydride, 4,4‘-(Hexafluoroisopropylidene)diphthalic Anhydride, and 3,3‘,4,4‘-Biphenylene Dianhydride: Structure?Viscosity Relationship.” Macromolecules 36 18 (2003): 6780–6790.
[3] MARYANNE MORES Patrick E C. Polyimidines. XI. Polyimidines from 3,3′,4,4′-benzophenonetetracarboxylic dianhydride and 4,4′-(hexafluoroisopropylidene) diphthalic anhydride[J]. Journal of Polymer Science Part A: Polymer Chemistry, 1990, 28 4: 811-823. DOI:10.1002/pola.1990.080280410.
[4] HUI WU Hezhou L Hua Li. Synthesis and Properties of a High-Molecular-Weight Polyimide Based on 4, 4’-(hexafluoroisopropylidene) Diphthalic Anhydride[J]. Advanced Materials Research, 2012, 35 1: 742-746. DOI:10.4028/www.scientific.net/AMR.550-553.742.
[5] XU LIU. Molecular design of free volume structure in thermally rearranged polybenzoxazole membranes for gas separation studied by positron annihilation[J]. Journal of Polymer Science, 2024. DOI:10.1002/pol.20240229.
[6] GAOJIE WU. Design and preparation of thermoplastic polyimides with high transmittance based on 4,4′-(4,4′-isopropyldiphenoxy)bis(phthalic anhydride) (BPADA)[J]. European Polymer Journal, 2023. DOI:10.1016/j.eurpolymj.2022.111798.
[7] YOUNG NAM KIM, YONG CHAE JUNG* Challenge for Trade-Off Relationship between the Mechanical Property and Healing Efficiency of Self-Healable Polyimide[J]. ACS Applied Materials & Interfaces, 2023. DOI:10.1021/acsami.3c12594.
4,4 '- (гексафторизопропилиден) дифталевого ангидрида запасные части и сырье
4,4 '- (гексафторизопропилиден) дифталевого ангидрида поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-021-33561011 +8613818804961 |
China | 402 | 58 | ||
| +86-17821320848; +8617821320848 |
China | 179 | 58 | ||
| +86-18222266713 +86-18222272656 |
China | 65 | 58 | ||
| +862156762820 +86-13564624040 |
China | 7707 | 58 | ||
| +86-37155567971 +86-13633837469 |
China | 5996 | 58 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-57156122185 +86-15967590394 |
China | 400 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 |