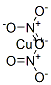
Copper dinitrate
- русский язык имя
- английское имяCopper dinitrate
- CAS №3251-23-8
- CBNumberCB1219900
- ФормулаCuN2O6
- мольный вес187.56
- EINECS221-838-5
- номер MDLMFCD00010967
- файл Mol3251-23-8.mol
| Температура плавления | 115°C |
| плотность | 1.00 g/mL at 20 °C |
| давление пара | 0Pa at 25℃ |
| растворимость | soluble in dioxane; reacts with ethyl ether |
| форма | blue-green orthorhombic crystals |
| цвет | blue-green orthorhombic crystals, crystalline; hygroscopic |
| Растворимость в воде | Soluble |
| Мерк | 13,2671 |
| Стабильность | Stable. Oxidant. Incompatible with combustible materials. |
| Surface tension | 73.2mN/m at 1.3g/L and 20.2℃ |
| Справочник по базе данных CAS | 3251-23-8(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | CUPRIC NITRATE |
| FDA UNII | 9TC879S2ZV |
| Система регистрации веществ EPA | Cupric nitrate (3251-23-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | O,C,Xi,N,Xn | |||||||||
| Заявления о рисках | 8-20/21/22-34-22-36/38-20-45-51/53-41-38-50/53-37/38-52/53 | |||||||||
| Заявления о безопасности | 53-17-26-36/37/39-45-36-61-39 | |||||||||
| РИДАДР | UN 3085 5.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| Класс опасности | 5.1 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 3251-23-8(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H318:При попадании в глаза вызывает необратимые последствия.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H272:Окислитель; может усилить возгорание.
-
оператор предупредительных мер
P220:Не допускать соприкосновения с одеждой и другими горючими материалами.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Copper dinitrate химические свойства, назначение, производство
Химические свойства
Cupric nitrate is a Blue crystalline solid.Использование
Light-sensitive papers; analytical reagent; mordant in textile dyeing; nitrating agent; insecticide for vines; coloring copper black; electroplating; production of burnished effect on iron; paints; varnishes, enamels; pharmaceutical preparations; catalyst.Определение
ChEBI: An inorganic nitrate salt having copper(2+) as the couterion.Общее описание
Obtained as a trihydrate and as a hexahydrate. Both are blue crystalline solids. Used in medicine, as an insecticide, in chemical analysis, in making light sensitive papers. Toxic oxides of nitrogen are produced in fires involving Copper dinitrate.Реакции воздуха и воды
Deliquescent. Water soluble.Профиль реактивности
Mixtures of Copper dinitrate with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride or other reducing agents may react explosively [Bretherick, 1979 p. 108-109]. A finely divided mixture of potassium ferrocyanide and Copper dinitrate exploded when dried at 220°C [Chem. Abst. 77:1343 (1972)]. Noncombustible, but Copper dinitrate will accelerate the burning of combustible materials. If large quantities of the material are involved in a fire or the material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion.Опасность
Oxidizer, causes violent combustion or explosion with organic materials.Угроза здоровью
Inhalation causes irritation of throat and lungs. Ingestion of large amounts causes violent vomiting and purging, intense pain, collapse, coma, convulsions, and paralysis. Solutions irritate eyes; contact with solid causes severe eye surface injury and skin irritation.Профиль безопасности
Moderately toxic by ingestion. A severe eye and skin irritant. Potentially explosive reaction above 22OOC with ammonium or potassium hexacyanoferrate(I1). Reaction with ammonia + potassium amide gives explosive product. Violent reaction with acetic anhydride. May ignite on prolonged contact with paper. Concentrated solutions may ipte in contact with tin or aluminum foil. Used as a fungicide, herbicide, and as a catalyst component in solid rocket fuel. When heated to decomposition it emits toxic fumes of NOx. See also COPPER COMPOUNDS and NITRATES.Возможный контакт
Cupric nitrate is used as an insecticide, in paint, varnish, enamel, and in wood preservatives. Metal compounds are often used in “hot” operations in the work-place. These may include, but are not limited to, welding, brazing, soldering, plating, cutting, and metallizing. At the high temperatures reached in these operations, metals often form metal fumes which have different health effects and exposure standards than the original metal compound and require specialized controls.Перевозки
UN1479 Oxidizing solid, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, Technical Name Required.Методы очистки
Crystallise it from weak aqueous HNO3 (0.5mL/g) by cooling from room temperature. The anhydrous salt can be prepared by dissolving copper metal in a 1:1 mixture of liquid NO2 and ethyl acetate and purified by sublimation [Evans et al. J Chem Soc, Faraday Trans 1 75 1023 1979]. The hexahydrate dehydrates to the trihydrate at 26o, and the anhydrous salt sublimes between 150 and 225o, but melts at 255-256o and is deliquescent.Несовместимости
A strong oxidizer. Aqueous solution is acidic; incompatible with bases. Violent reaction with potassium hexacyanoferrate; ammonia and potassium amide mixtures; acetic anhydrides, cyanides, ethers. Forms explosive materials with nitromethanes, sodium hypobromite; acetylene; chemically active metals, such as potassium, sodium, etc. May ignite on contact with aluminum foil or tin. Risk of spontaneous combustion with combustibles (wood, cloth, etc.) organics, or reducing agents and readily oxidizable materials. Attacks metals in the presence of moisture.Утилизация отходов
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill. Add slowly to water; stir in excess soda ash. Let stand, then neutralize. Decant solution and flush to sewer; landfill sludgeCopper dinitrate запасные части и сырье
Copper dinitrate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| 0082-42-721-7177 | South Korea | 297 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 |