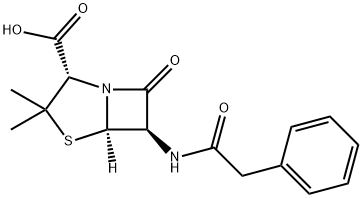
Penicillin G
- русский язык имя
- английское имяPenicillin G
- CAS №61-33-6
- CBNumberCB1211939
- ФормулаC16H18N2O4S
- мольный вес334.39
- EINECS200-506-3
- номер MDLMFCD00069665
- файл Mol61-33-6.mol
| Температура плавления | 82-83 °C |
| Температура кипения | 663.3±55.0 °C(Predicted) |
| альфа | D +282° (ethanol) |
| плотность | 1.2729 (rough estimate) |
| показатель преломления | 1.6930 (estimate) |
| температура хранения | 2-8°C |
| растворимость | H2O: 100 mg/mL |
| форма | powder |
| пка | 2.45±0.50(Predicted) |
| цвет | Crystals |
| Растворимость в воде | 2.675g/L(25 ºC) |
| Справочник по базе данных CAS | 61-33-6(CAS DataBase Reference) |
| FDA UNII | Q42T66VG0C |
| Код УВД | J01CE01,S01AA14 |
| Система регистрации веществ EPA | 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]- (2S,5R,6R)- (61-33-6) |
| Коды опасности | Xn |
| Заявления о рисках | 42/43 |
| Заявления о безопасности | 36/37 |
| WGK Германия | 2 |
| RTECS | XH9700000 |
| F | 10-23 |
| кода HS | 32041900 |
| Банк данных об опасных веществах | 61-33-6(Hazardous Substances Data) |
| Токсичность | LD50 orl-rat: 8 g/kg ANTCAO 12,249,62 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
P362+P364:Снять всю загрязненную одежду и выстирать ее перед повторным использованием.
Penicillin G химические свойства, назначение, производство
Описание
Penicillin was the first natural antibiotic used to treat bacterial infections and continues to be one of the most important antibiotics.The name comes from the fungus genus Penicillium from which it was isolated. Penicillus is Latin for brush and refers to the brushlike appearance of filamentous Penicillium species.Species of this genus are quite common and appear as the bluish-green mold that appears on aged bread, fruit, and cheese. The term penicillin is a generic term that refers to a number of antibiotic compounds with the same basic structure. Therefore it is more appropriate to speak of penicillins than of penicillin.The general penicillin structure consists of a β-lactam ring and thiazolidine ring fused together with a peptide bonded to a variable R group. Penicillin belongs to a group of compounds called β-lactam antibiotics. This in turn inhibits the formation of peptidoglycan cross-links in bacteria cell walls.
История
The discovery of penicillin is generally credited to Alexander Fleming (1881 1955) in 1928, but the development of penicillin as an antibiotic took place sporadically over the last decades of the 19th century and first half of the 20th century. Mass production of penicillin by U.S.firms started in 1943,and it was used immediately to treat wounded soldiers. Penicillin reduced suff ering, prevented amputations,cured pneumonia,and saved thousands of lives during the war and was hailed as a miracle drug. The United States increased production throughout the war years and after the war widespread civilian use commenced. Fleming, Florey,and Chain shared the Nobel Prize in physiology or medicine in 1945 for their work on penicillin.Использование
Antibacterial.Показания
Benzylpenicillin or penicillin G has a narrow antimicrobial spectrum. It is active with respect to Gram-positive bacteria (staphylococcus, streptococcus, and pneumococci), causative agent of diphtheria, and anthrax bacillus. Gram-negative bacteria are resistant to it. Benzylpenicillin is broken down by stomach acid and destroyed by staphylococcus penicillinase.Приобретенная устойчивость
Staph. aureus was originally highly susceptible to benzylpenicillin, but at least 85–90% of clinical isolates are now β-lactamase-producing strains. Most clinical isolates of coagulase- negative staphylococci are also resistant. β-Lactamaseproducing strains of E. faecalis produce an enzyme identical to staphylococcal penicillinases, but these strains are increasingly uncommon. The emergence of penicillin-resistant staphylococci, enterococci and pneumococci, due to the acquisition of mosaic PBPs with decreased binding affinity for penicillin, has been described worldwide. Most strains of penicillin- resistant Gram-positive clinical isolates also demonstrate reduced susceptibility to other β-lactam agents but, in rare cases, cross-resistance is not seen for all cephalosporins.Strains of N. gonorrhoeae for which the MIC of benzylpenicillin increased from 0.06 mg/L to >2 mg/L appeared in the 1970s, as the result of the production of modified PBPs with reduced affinity for β-lactam antibiotics; fully resistant strains producing TEM-1 β-lactamase also emerged. Currently penicillin resistance occurs in more than 60% of isolates in some parts of the world.
Контактные аллергены
Benzyl penicillin is actually used only intravenously. It was formerly a frequent cause of contact allergy in health care workers. Facial contact dermatitis was recently reported in a nurse.Клиническое использование
For years, the most popular penicillin has been penicillin G,or benzylpenicillin. In fact, with the exception of patients allergicto it, penicillin G remains the agent of choice for thetreatment of more different kinds of bacterial infection thanany other antibiotic. It was first made available as the watersolublesalts of potassium, sodium, and calcium. These saltsof penicillin are inactivated by the gastric juice and are noteffective when administered orally unless antacids, such ascalcium carbonate, aluminum hydroxide, and magnesiumtrisilicate; or a strong buffer, such as sodium citrate, isadded. Also, because penicillin is absorbed poorly from the intestinal tract, oral doses must be very large, about fivetimes the amount necessary with parenteral administration.Only after the production of penicillin had increased enoughto make low-priced penicillin available did the oral dosageforms become popular. The water-soluble potassium andsodium salts are used orally and parenterally to achieve highplasma concentrations of penicillin G rapidly. The morewater-soluble potassium salt usually is preferred when largedoses are required. Situations in which hyperkalemia is adanger, however, as in renal failure, require use of thesodium salt; the potassium salt is preferred for patients onsalt-free diets or with congestive heart conditions.Профиль безопасности
Poison by ingestion, intravenous,intracerebral, intraspinal, subcutaneous, and possibly otherroutes. Human (child) systemic effects by parenteral route:changes in cochlear (inner ear) structure or function,convulsions, and dyspnea. Questionable carcinPenicillin G запасные части и сырье
сырьё
запасной предмет
Penicillin G поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8617320513646 | China | 9606 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +8615102730682 | CHINA | 566 | 55 | |
| +8618523575427 | China | 49732 | 58 | |
| +8618633865755 | CHINA | 955 | 58 | |
| +8613367258412 | China | 10319 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| 15369953316 +8615369953316 |
China | 2123 | 58 | |
| +8615255079626 | China | 23541 | 58 |
Penicillin G Обзор)
1of4