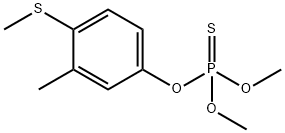
Фентион
- английское имяFenthion
- CAS №55-38-9
- CBNumberCB1129166
- ФормулаC10H15O3PS2
- мольный вес278.33
- EINECS200-231-9
- номер MDLMFCD00055449
- файл Mol55-38-9.mol
| Температура плавления | 7.5°C |
| Температура кипения | 87°C (0.01 mmHg) |
| плотность | 1.25 |
| давление пара | 7.4 x 10-4 Pa (20 °C) |
| показатель преломления | nD20 1.5698 |
| Fp | >100 °C |
| температура хранения | 2-8°C |
| растворимость | Chloroform (Sparingly), DMSO (Sparingly) |
| форма | liquid |
| Растворимость в воде | 0.0055 g/100 mL |
| Мерк | 13,4030 |
| БРН | 1974129 |
| Пределы воздействия | OSHA PEL: TWA 0.2 mg/m3; ACGIH TLV: TWA 0.2 mg/m3 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| Справочник по базе данных CAS | 55-38-9(CAS DataBase Reference) |
| FDA 21 CFR | 556.280 |
| Рейтинг продуктов питания EWG | 1-3 |
| FDA UNII | BL0L45OVKT |
| Справочник по химии NIST | Fenthion(55-38-9) |
| Система регистрации веществ EPA | Fenthion (55-38-9) |
| Пестициды Закон о свободе информации (FOIA) | Fenthion |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T;N,N,T,Xn,F |
| Заявления о рисках | 21/22-23-48/25-50/53-68-67-65-38-11 |
| Заявления о безопасности | 36/37-45-60-61-62 |
| РИДАДР | UN 2810/3017 |
| WGK Германия | 3 |
| RTECS | TF9625000 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| кода HS | 29309090 |
| Банк данных об опасных веществах | 55-38-9(Hazardous Substances Data) |
| Токсичность | LD50 in male, female rats (mg/kg): 215, 245 orally (Gaines) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H330:Смертельно при вдыхании.
H301+H311:Токсично при проглатывании или при контакте с кожей.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
Фентион химические свойства, назначение, производство
Описание
Fenthion is a colorless oily liquid (technical grade, brown oily liquid with a mercaptan-like odor).Химические свойства
Pure fenthion is a colorless liquid. Technical fenthion is a yellow or brown oily liquid with a weak garlic odor. It is insoluble or very sparingly soluble in water, but soluble in all organic solvents, alcohols, ethers, esters, halogenated aromatics, and petroleum ethers. It is grouped by the US EPA under RUP and hence requires handling by qualifi ed, certifi ed, and trained workers. Fenthion is used for the control of sucking and biting pests, i.e., fruit fl ies, stem borers, mosquitoes, and cereal bugs. In mosquitoes, it is toxic to both the adult and immature forms (larvae). The formulations of fenthion include dust, emulsifi able concentrate, granular, liquid concentrate, spray concentrate, ULV, and wettable powder.Использование
Fenthion is an organothiophosphate used as an insecticide. Fenthion is also a potent acaricide.Определение
ChEBI: An organic thiophosphate that is O,O-dimethyl hydrogen phosphorothioate in which the hydrogen atom of the hydroxy group is replaced by a 3-methyl-4-(methylsulfanyl)phenyl group. It exhibits acaricidal and insecticidal activities.Общее описание
Yellow to tan oily liquid with a slight odor of garlic.Профиль реактивности
Fenthion may react with strong reducing agents such as hydrides to generate highly toxic and flammable phosphine gas. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.Опасность
Toxic by ingestion, inhalation, and skin absorption; use may be restricted, cholinesterase inhibitor. Toxic by skin absorption; questionable carcinogen.Угроза здоровью
Fenthion is moderately toxic to mammals, and highly toxic to birds. Exposures to fenthion cause poisoning with symptoms among occupational workers as observed with organophosphate pesticide-induced toxicity. These include, but are not limited to, numbness, tingling sensations, incoordination, headache, dizziness, tremor, nausea, abdominal cramps, sweating, blurred vision, diffi culty breathing or respiratory depression, and slow heart beat. Very high doses may result in unconsciousness, incontinence, and convulsions or fatality. Reports have indicated that exposures to fenthion cause adverse effects on the central and peripheral nervous systems, and the heart of exposed workers.Пожароопасность
Fenthion is probably combustible.Сельскохозяйственное использование
Insecticide, Larvicide, Veterinary medicine: Not registered for use in the U.S. Not approved for use in EU countries. Used in 20 countries including Australia. There are 23 global suppliers.Торговое название
B 29493®; BACID®; BAY 29493®; BAYCID®; BAYER 29493®; BAYER 9007®; BAYER S-1752®; BAYTEX®; ENTEX®; LEBAYCID®; MPP®; MPP (pesticide) OMS 2®; PHENTHION®; PILARTEX®; QUELETOX®; S 1752®; SPOTTON®; SULFIDOPHOS®; TALODEX®; TIGUVON®Возможный контакт
A potential danger to those involved in the manufacture, formulation, or application of this agricultural chemical and pesticide; insecticideЭкологическая судьба
Biological. From the first-order biotic and abiotic rate constants of fenthion in estuarine water and sediment/water systems, the estimated biodegradation half-lives were 22.4 and 3.9–14.5 days, respectively (Walker et al., 1988).Surface Water. In estuarine water, the half-life of fenthion was 4.6 days (Lacorte et al., 1995).
Plant. In plants, fenthion oxidizes to the mesulfenfos and sulfone which further degrades to the sulfone phosphate before undergoing hydrolysis (Hartley and Kidd, 1987).
Photolytic. Fenthion was oxidized to the corresponding sulfoxide and trace amounts (<5% yield) of sulfone when sorbed on soil and exposed to sunlight. The photosensitized oxidation was probably due to the presence of singlet oxygen. The degradation rate was higher in soils containing the lowest organic carbon (Gohre and Miller, 1986).
Chemical/Physical. Stable at temperatures below 210°C (Worthing and Hance, 1991). Emits very toxic fumes of phosphorus and sulfur oxides when heated to decomposition (Sax and Lewis, 1987; Lewis, 1990).
Метаболический путь
The major route of fenthion metabolism is by oxidation to the sulfoxide which has a higher insecticidal activity than the parent compound. The subsequent oxidation of the sulfoxide to fenthion sulfone, the biological activity of which is considerably lower, is slow in plants but of major significance in animals. An additional route of bioactivation is through oxidative desulfuration to form fenoxon and, in all, five oxidative metabolites (fenthion sulfoxide, fenthon sulfone, fenoxon, fenoxon sulfoxide and fenoxon sulfone) have been detected in most biological systems. Hydrolysis, in contrast to most insecticidal phosphorothioates, appears to proceed via direct hydrolysis of fenthion rather than its oxon. The phenolic leaving group (3-methyl-4-thiomethylphenol) is identical to that of fenamiphos and although no studies have described the fate of the phenolic moiety for fenthion, it is likely that it will be metabolised by similar routes to those described for fenamiphos.Перевозки
UN3018 Organophosphorus pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, alkaline insecticides. Organothiophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrideds and active metals. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.Утилизация отходов
Hydrolysis and landfill for small quantities; incineration with flue gas scrubbing for large amounts. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA officeФентион запасные части и сырье
сырьё
1of2
запасной предмет
Фентион поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615604608665 15604608665 |
CHINA | 9414 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 |