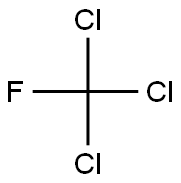
Фтортрихлорметан
- английское имяTrichlorofluoromethane
- CAS №75-69-4
- CBNumberCB1127379
- ФормулаCCl3F
- мольный вес137.37
- EINECS200-892-3
- номер MDLMFCD00000784
- файл Mol75-69-4.mol
| Температура плавления | -111°C |
| Температура кипения | 23.8°C |
| плотность | 1.494 |
| плотность пара | 5.04 (vs air) |
| давление пара | 12.85 psi ( 20 °C) &_& 39.17 psi ( 55 °C) |
| показатель преломления | 1.382 |
| Fp | 2 °C |
| температура хранения | 2-8°C |
| растворимость | water: soluble1g/L |
| цвет | Colorless, odorless liquid |
| Запах | Odorless; weak chlorinated solvent. |
| Растворимость в воде | insoluble. 0.124 g/100 mL |
| Мерк | 13,9714 |
| БРН | 1732469 |
| констант закона Генри | At 25 °C: 88.2 and 123 in distilled water and seawater, respectively (Hunter-Smith et al., 1983) |
| Пределы воздействия | NIOSH REL: ceiling 1,000 ppm (5,600 mg/m3), IDLH 2,000 ppm; OSHA PEL: TWA 1,000 ppm; ACGIH TLV: ceiling 1,000 ppm (adopted). |
| Диэлектрическая постоянная | 3.1(21℃) |
| Стабильность | Stable. Incompatible with bronze, silver, copper, beryllium, alkali and alkaline earth metals. |
| ИнЧИКей | CYRMSUTZVYGINF-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 75-69-4(CAS DataBase Reference) |
| FDA 21 CFR | 701.30 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 990TYB331R |
| Справочник по химии NIST | Trichloromonofluoromethane(75-69-4) |
| Система регистрации веществ EPA | CFC-11 (75-69-4) |
| UNSPSC Code | 12142201 |
| NACRES | NA.24 |
| Коды опасности | Xn;N,Xi,N,Xn,T,F | |||||||||
| Заявления о рисках | 20-59-23-21/22-36/37/38-20/22-11-23/25-39/23/24/25-23/24/25-21-67-66-36 | |||||||||
| Заявления о безопасности | 23-24/25-59-61-45-36/37-36-26-16-24-7 | |||||||||
| РИДАДР | 3082 | |||||||||
| OEL | Ceiling: 1000 ppm (5600 mg/m3) | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | PB6125000 | |||||||||
| Примечание об опасности | Irritant | |||||||||
| Банк данных об опасных веществах | 75-69-4(Hazardous Substances Data) | |||||||||
| Токсичность | Drinking water standard: No MCLGs or MCLs have been proposed although trichlorofluoromethane has been listed for regulation (U.S. EPA, 1996). A DWEL of 100 μg/L was recommended (U.S. EPA, 2000). | |||||||||
| ИДЛА | 2,000 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H302:Вредно при проглатывании.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H336:Может вызывать сонливость или головокружение.
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
H331:Токсично при вдыхании.
H420:Разрушает озоновый слой.
-
оператор предупредительных мер
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P502:Обратиться к изготовителю или поставщику для получения информации о вторичной переработке или утилизации.
Фтортрихлорметан химические свойства, назначение, производство
Описание
Trichlorofluoromethane is a chlorofluorocarbon (CFC) which is commonly used as a refrigerant, a foaming or blowing agent in industry, a solvent, an aerosol propellant, and in chemical syntheses.Trichlorofluoromethane is a colorless, odorless gas at normal temperatures and pressures. Under high pressures as in cans, tanks or refrigerators it is in liquid form. When released from a pressurized container it evaporates almost instantly and can cause freezing at the point of release. At very high concentrations in air it may smell like ether. It is slightly soluble in water but evaporates quickly if exposed to air. The chemical formula for Trichlorofluoromethane is CFCL3.
Химические свойства
Fluorotrichloromethane is a colorless liquid or gas. Chlorinated solvent odor. The Odor Threshold is 5.0 ppm.Вхождение
Trichlorofluoromethane is man-made and its presence in the environment is due to releases from common household and industrial uses. It is extremely stable in the atmosphere and does not degrade naturally. It can also be produced as an industrial by-product wherever chlorine products are used; including small quantities which are formed by reaction of chlorine disinfectants with organic pollutants in water. It is released into the air by leaking refrigeration units and air conditioners and by spray paint, spray varnish, spray cosmetics and other sprays in which it has been used as a propellant. Since 1978 many uses of trichlorofluoromethane as a propellant have been prohibited by law in the U.S. It can still be used as a propellant in some specialized products used in businesses and industries. It may also be found in air emissions and waste waters from a number of industries particularly refrigeration, electronics and foam manufacturing.Использование
Trichlorofluoromethane is a coolant in conditioning systems. It is a bulking agent for polymer foams. It is also used as a degrease solvent for printed circuit boards and to dry clean textiles. It is used as an aerosol propellant in various industries.Определение
ChEBI: A one-carbon compound that is methane in which the hydrogens have been replaced by three chlorine and one fluorine atom.Общее описание
A clear light colored liquid. Nearly odorless. Denser than water. Poses low acute health hazard to humans. Primary hazard is to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways.Реакции воздуха и воды
Water soluble. Hydrolyzed slowly.Профиль реактивности
Trichlorofluoromethane is incompatible with alkali or alkaline earth metals, powdered aluminum, zinc and beryllium. Trichlorofluoromethane reacts violently with barium and lithium.Угроза здоровью
Breathing concentrations approaching 10% in air will cause dizziness and drowsiness. Contact with tissues may cause frostbite.Пожароопасность
Special Hazards of Combustion Products: Produces irritating and toxic products when heated to decomposition temperatures.Химическая реактивность
Reactivity with Water: No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Профиль безопасности
High concentrations cause narcosis and anesthesia in humans. Human systemic effects by inhalation: conjunctiva irritation, fibrosing alveolitis, and liver changes. Experimental poison by inhalation. Moderately toxic by intraDeritoneal route. Reacts violentlv with I aluminum, barium, or lithium. When heated to decomposition it emits highly toxic fumes of Fand Cl-. Used as an aerosol propellant, refrigerant, and blowing agent for polymeric foams. See also CHLORINATED HYDROCARBONS, ALIPHATIC; and FLUORIDES.Возможный контакт
This material is used as a refrigerant; aerosol propellant; and foaming agent; as blowing agent in production of polyurethane foams.Канцерогенность
When administered by gavage to groups of 50 male and 50 female mice at daily doses of 1962 or 3952mg/kg, 5 days/week for 78 weeks followed by a 13week observation period, there was no evidence of carcinogenicity. Also,when given to rats at daily doses of 488 and 977mg/kg for males and 538 and 1077mg/kg for females, again for 5 days/week for 78 weeks, but followed by a 28–33 week observation period, there was no evidence of carcinogenicity. Maltoni et al. exposed groups of 90 male and 90 female Sprague–Dawley rats and groups of 60 male and 60 female Swiss mice by inhalation to levels of 1000 and 5000 ppm 4 h/day, 5 days/week for life. The exposures did not produce evidence of carcinogenicity.Экологическая судьба
Biological. In a static-culture-flask screening test, trichlorofluoromethane was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum. No significant degradation was observed after 28 d of incubation. At substrate concentrations of 5 and 10 mg/L, percent losses due to volatilization were 58 and 37% after 10 d (Tabak et al., 1981).Chemical/Physical. When trichlorofluoromethane (50 μg/L) in an ultrasonicator was exposed to 20-kHz ultrasound at 5 °C, nearly 100% degradation was achieved after 6 min. During sonication, the pH of the aqueous solution decreased, which is consistent with the formation of HCl, hydrofluoric acid, and acidic species from fluorine and chlorine. In this experiment <5% of trichlorofluoroethane was lost to volatilization (Cheung and Kurup, 1994).
Перевозки
Hazard Class: 2.2; Labels: 2.2-Nonflammable compressed gas, Technical Name Required.Несовместимости
Chemically active and powdered metals: aluminum, barium, sodium, potassium, calcium, powdered aluminum; zinc, magnesium.Утилизация отходов
Incineration, preferably after mixing with another combustible fuel. Care must be exer cised to assure complete combustion to prevent the forma tion of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regu latory agencies for guidance on acceptable disposal prac tices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations govern ing storage, transportation, treatment, and waste disposal.Фтортрихлорметан запасные части и сырье
Фтортрихлорметан поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 400-1166-196 18981987031 |
China | 11707 | 57 | |
| 400-1166-196 13458535857 |
China | 13350 | 58 | |
| 400-1166-196 18502815961 |
China | 14603 | 60 |