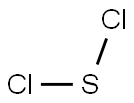Why is SCl2 polar?
Sulfur dichloride (SCl2) is a polar molecule. Chlorine is more electronegative than sulfur, which means it will attract the shared electrons in the S-Cl bond more strongly. SCl2 has a bent or angular geometry due to the presence of two lone pairs on the sulfur atom.
The formation of a polar molecule is caused by the geometrical structure and the difference in electronegativity value of atoms in the SCl2 molecule.

Due to the existence of two lone pairs on the Sulfur atom, the Sulfur dichloride (SCl2) molecule has a twisted V- shape bent form. According to the VSEPR hypothesis, lone pairs and bond pairs repel each other, causing the S-Cl bonds to move the lower side of the molecular structure, resulting in a V-shaped molecule.
Secondly, the difference between the electronegativity of sulfur and chlorine atoms makes the S-Cl bonds polar and as a result, the entire molecule also becomes polar and gives a net dipole moment of 0.54D.
Because of the asymmetric shape of the SCl2 molecule, the charge is dispersed non-uniformly among the sulfur and chlorine atoms, resulting in the formation of positive and negative poles across the SCl2 molecule.