Synthesis of Coblopasvir Hydrochloride
Introduction of Coblopasvir Hydrochloride
Coblopasvir hydrochloride (KW-136) is an antiviral agent developed by Beijing Kawin Technology Share-Holding for the treatment of hepatitis C virus (HCV) infection. The compound, which is a potent pangenotypic inhibitor of HCV nonstructural protein 5A (NS5A), exhibited picomolar antiviral activity against HCV replicons across a variety of genotypes. In February 2020, the Chinese National Medical Products Administration (CNMPA) approved coblopasvir in combination with sofosbuvir for the treatment of HCV.
Synthesis of Coblopasvir Hydrochloride
Coblopasvir Hydrochloride is synthesised using catechol as raw material, Coblopasvir Dibromide is synthesised first and then through a series of chemical reactions such as coupling and refluxing. The specific synthesis method is as follows:
Step 1: Synthesis of Coblopasvir Dibromide
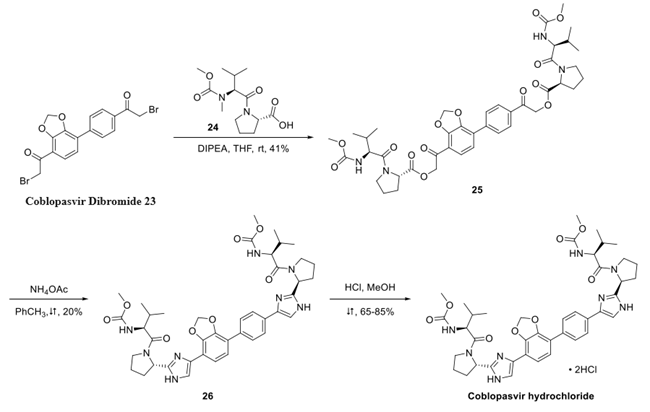
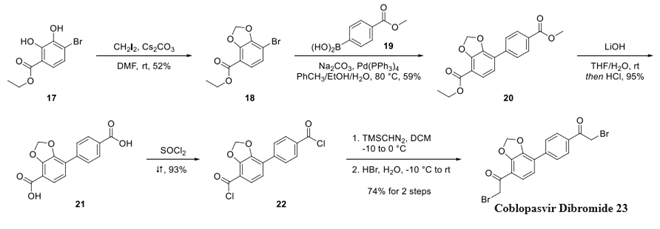
A synthetic process for the preparation of a novel crystalline form of coblopasvir hydrochloride (designated as Form H) was described by Yang and Yan. Strategically, the compound’s assembly necessitated the construction of a diaryl core with peripheral α-bromoketones at each terminus of the synthon followed by double displacement of both bromides with an amino acid derivative. The synthesis of dibromide 23 began with the reaction of catechol 17 with methylene iodide under basic conditions to form acetal 18, followed by Suzuki coupling with boronic acid 19 to form biphenyl 20. Saponification followed by acid chloride formation preceded a modified Niernestein reaction, which converted the bis-acid chlorides to the bis-α-haloketones. Treatment with aqueous hydrobromic acid furnished dibromide 23.
Step 2: Preparation of Coblopasvir Hydrochloride
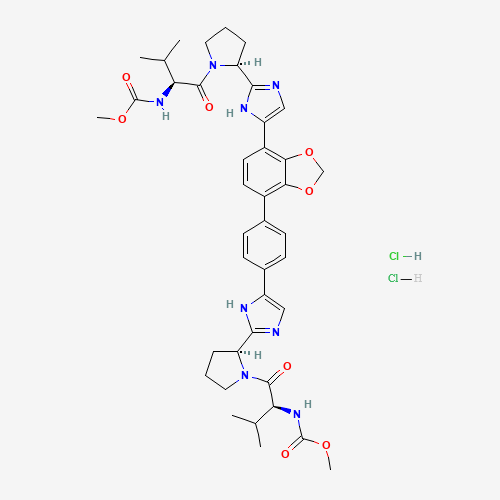
Dibromide 23 was coupled with dipeptide 24 to form the bis-carbamate 25. The reaction of 25 with ammonium acetate in refluxing toluene constructed the two imidazole rings and afforded the free base of coblopasvir 26, albeit in low yield. Extensive screening of salt-formation conditions resulted in the identification of methanol as the solvent of choice to provide the appropriate solid form of the drug. When free base 26 was treated with methanolic HCl at 60−65 °C, coblopasvir hydrochloride was isolated in 65− 86% yield.