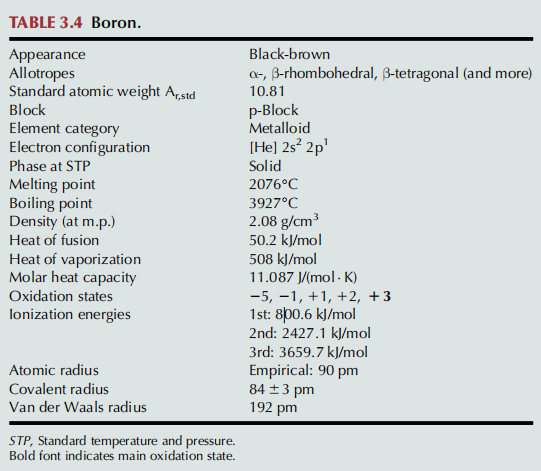
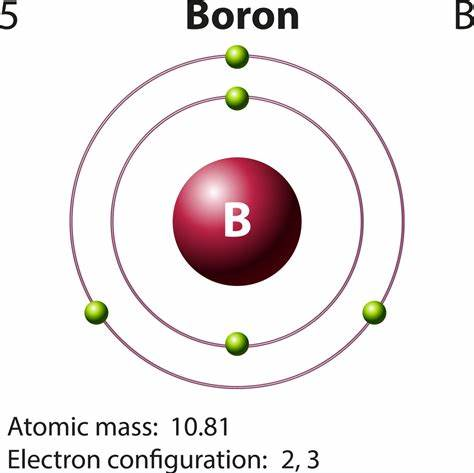
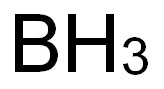Why is Boron green in a flame?
In a flame test, the element Boron emits EM radiation that is predominantly green in color.

As the electrons return to their original energy levels, they release the excess energy in the form of light. In the case of boron compounds, this light is predominantly in the green part of the spectrum, resulting in the bright green color seen in fireworks displays.
Boron and its compounds have very little such use in fireworks. Elemental boron can be burned for green flame, but elemental boron is too expensive for practicable fireworking. Similarly for boranes. Borax is cheap, but its sodium content swamps with yellow the green it might contribute to a flame, and it being a decahydrate makes its principal use in fireworking one of an inhibitor. Boric acid may be dissolved in methanol or other fuels for air burning; I’ve done this to make a green methyl alcohol flame. However, the main use of boric acid in solids in fireworking is as a source of acidity to inihibit alkaline aluminum reactions.
You may like
Related articles And Qustion
See also
Lastest Price from Boron manufacturers

US $10.00-7.00/kg2025-02-13
- CAS:
- 7440-42-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 tons

US $0.00-0.00/g2024-09-25
- CAS:
- 7440-42-8
- Min. Order:
- 50g
- Purity:
- 99%
- Supply Ability:
- 60kg