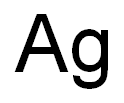Flame test and chemical principles of silver

Chemistry principles
The flame test is one of the most commonly used analytical processes in chemistry. It is widely used to detect and analyze the presence of certain elements in the given salt or compound. Primarily, the flame test detects the presence of metal ions in a compound.
The chemistry behind the flame test is simple. As we know that when an atom or ion is excited by heating to high temperatures, the electrons are promoted from their normal unexcited state into other orbitals, known as higher orbitals, as they have higher energy as compared to the normal or ground state orbitals.
When these excited electron falls back down to lower levels which can happen simultaneously or in several steps, the energy they have absorbed is released. This energy is released in the form of light.
Each jump involves the release of a specific amount of energy in the form of light. And each transition from higher to lower orbital corresponds to a frequency or wavelength.
All these jumps or transitions result in the formation of a spectrum of lines. Some of these lines are part of the visible part of the spectrum.
And the final color we see is a combination of all these individual colors. This color is the distinctive color of the element we observe in the flame test.
Flame test for silver
Silver do not produce a characteristic flame test color. There are several possible explanations for this, one being that the thermal energy isn't sufficient to excite the electrons of these elements enough to release energy in the visible range.
You may like
Related articles And Qustion
See also
Lastest Price from Silver manufacturers

US $6.00/kg2025-04-21
- CAS:
- 7440-22-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $4.00/KG2025-03-28
- CAS:
- 7440-22-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20TONS