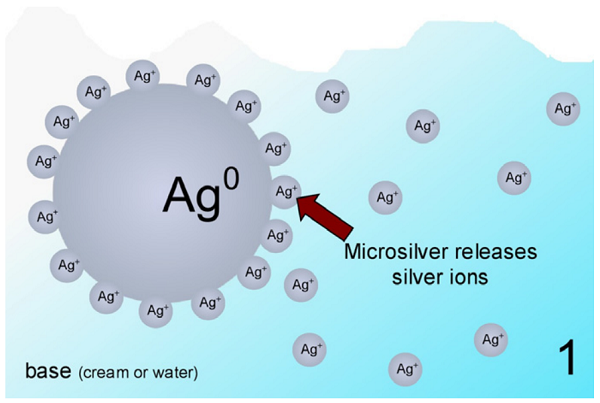
What is the charge of silver?
Silver typically has a +1 charge when involved in an ionic bond.
Although silver can form both +1 and +2 cations, the +2 is so rare that we usually name Ag + as silver ion, not silver(I) ion. Ag 2 + is named silver(II) ion. We will assume that all of the metallic elements other than those mentioned above can have more than one charge, so their cation names will include a Roman numeral.
When atoms form ionic bonds, they can either lose of gain electrons depending on which would make them stable. Acquiring stability is a result of having a full valence shell of electrons. Cations are formed when atoms lose electrons and anions are formed when atoms gain electrons.
Silver has an ionic charge of +1. You can look at the oxidation states on a more detailed periodic table. You could use context clues based off of what it is reacting with. You might also notice the trend for elements group 11 to have a +1 and elements in group 12 to have a +2.
You may like
Related articles And Qustion
See also
Lastest Price from Silver manufacturers

US $6.00/kg2025-04-21
- CAS:
- 7440-22-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $4.00/KG2025-03-28
- CAS:
- 7440-22-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20TONS