When DHA is applied on the skin surface
Chemical property
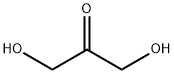
DHA is a 3-carbon keto sugar (1,3-dihydroxy-2-propanone), molecular weight 90.08, presenting as a white to off-white, fine crystalline powder with a faint characteristic odour. DHA is readily soluble in water or 70% ethanol. Its melting point is 68–71°C. For long-term conservation, it must be stored in a dry place below 6°C.
1,3-Dihydroxyacetone (DHA), the main active ingredient in sunless tanning skin-care preparations and an essential precursor for the synthesis of various fine chemicals, is produced on an industrial scale by microbial fermentation of glycerol (HY-B1659) in Gluconobacter oxydans. 1,3-Dihydroxyacetone is also used to synthesise new biodegradable polymers by combining with lactic acid (HY-B2227). It is a natural product found in Arabidopsis thaliana, Homo sapiens, and other organisms.
Uses
DHA was originally used as an artificial sweetener for diabetic patients. In the 1950s, it was also used orally for children with glycogen storage diseases when it was discovered that it induced a brown pigmentation to the skin. Currently, DHA is the only FDA-approved agent for sunless tanning. It has also been used in vitiligo to temporarily induce pigmentation in affected areas.
DHA is a chemical substance that binds the amino terminals of epidermal amino acids and proteins. Doing so imparts a brown colour to the skin, simulating a 'tanning response'. Of course, this is only a cosmetic dyeing reaction completely different from a true tanning reaction involving the increased production and transfer of melanosomes from melanocytes to keratinocytes. Nevertheless, the browning effect of DHA application can be helpful as a photoprotective tool against long-wave ultraviolet.
Mechanism of action
Once DHA has been applied to the skin surface, it reacts with the amino-terminal groups of the free amino acids and proteins in the epidermis. This leads to the formation of chromophores named 'melanoids', polymeric compounds which are linked by lysine side chains to the proteins of the stratum corneum: therefore, the action is limited to the upper layers of the skin. The orange-brown colouring develops within 3 to 10 hours after the application, and it is not removed from the surface by washing or swimming. It fades away only when the outer layers of the stratum corneum are shed during the natural desquamation process. The intensity of the colour, varying from light to dark orange-brown, depends on the concentration of DHA and on the frequency of its application: usually, 3-5 applications give rise to a golden-brown colour simulating a tanning reaction. Muizzuddin and co-workers, using three non-ionic oil-in-water cream emulsion formulations (containing respectively2.5%, 5% and 6.5% DHA concentrations), demonstrated a dose-response relationship between the concentration of DHA and the degree of skin darkening measured by chronometer at 5 and 18 h after the application.
References
[1] Monfrecola, G. and E. Prizio. “Chapter 25 - Self tanning."Comprehensive Series in Photosciences 3 (2001): 487, 489-493.
You may like
Related articles And Qustion
Lastest Price from 1,3-Dihydroxyacetone manufacturers

US $0.00/kg2025-11-28
- CAS:
- 96-26-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000 kg

US $5.00-0.50/KG2025-05-07
- CAS:
- 96-26-4
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS