1,3-Dihydroxyacetone: Biosynthesis and Detection Method
General Description
The biosynthesis of 1,3-Dihydroxyacetone using Gluconobacter oxydans involves the conversion of glycerol facilitated by enzymes like glycerol dehydrogenase and dihydroxyacetone kinase. Metabolic engineering enhances production by redirecting metabolic flux. Optimizing fermentation conditions and using a fed-batch strategy improve efficiency. Scaling up in bioreactors demonstrates industrial viability. In contrast, the electrochemiluminescence method with tris(2,2'-bipyridine)ruthenium(II) enables sensitive detection of 1,3-Dihydroxyacetone without chemical modifications. It offers a low detection limit of 1.79 μM, high sensitivity, and broad linear ranges, making it valuable in quality control for cosmetics and food industries.

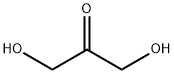
Figure 1. 1,3-Dihydroxyacetone
Biosynthesis
1,3-Dihydroxyacetone is a key organic compound with extensive applications in the cosmetics, pharmaceutical, and food industries due to its ability to prevent excessive water evaporation, along with providing anti-ultraviolet radiation protection and antioxidant properties. The biosynthesis of 1,3-Dihydroxyacetone, particularly using the bacterium Gluconobacter oxydans, represents a significant biotechnological advancement. The process of biosynthesizing 1,3-Dihydroxyacetone involves the fermentation of glycerol. Gluconobacter oxydans, a species known for its robust oxidative capabilities, is employed to convert glycerol into 1,3-Dihydroxyacetone through enzymatic reactions. In this bacterium, the oxidation of glycerol is facilitated by specific enzymes that include glycerol dehydrogenase and dihydroxyacetone kinase, which are crucial for the conversion process. In the context of enhancing the production levels of 1,3-Dihydroxyacetone, metabolic engineering plays a pivotal role. By selectively knocking out genes unrelated to 1,3-Dihydroxyacetone synthesis, researchers can redirect the metabolic flux towards increased 1,3-Dihydroxyacetone production. For instance, in one study, the elimination of certain dehydrogenase genes in Gluconobacter oxydans led to a substantial improvement in 1,3-Dihydroxyacetone yield. Moreover, optimizing the fermentation conditions is another critical aspect. Adjustments in the seed culture conditions, nutrient composition of the fermentation media, and the overall environmental conditions like pH and temperature can significantly impact the efficiency of 1,3-Dihydroxyacetone synthesis. The use of a fed-batch strategy, where substrates are added in controlled amounts during fermentation, helps maintain optimal metabolic activity and prevents substrate inhibition, leading to higher 1,3-Dihydroxyacetone concentrations. Finally, the scalability of the 1,3-Dihydroxyacetone production process in bioreactors demonstrates the industrial viability of this biosynthetic pathway. By fine-tuning both genetic and process engineering aspects, it is possible to achieve high yields of 1,3-Dihydroxyacetone, which are essential for meeting the demand from various industrial sectors. The development of such efficient biosynthetic strategies underscores the potential of microbial systems in sustainable chemical production. 1
Detection Method
The detection of 1,3-Dihydroxyacetone, a compound widely used in cosmetics and as a food additive, can be effectively conducted using an advanced electrochemiluminescence (ECL) technique involving tris(2,2'-bipyridine)ruthenium(II). This method capitalizes on the electrochemiluminescent properties of the ruthenium complex when used as a coreactant with 1,3-Dihydroxyacetone. In this detection approach, 1,3-Dihydroxyacetone enhances the electrochemiluminescence of Ru(bpy)₃²⁺, significantly more so than traditional coreactants like sodium oxalate. This increase in efficiency, about 25 times higher than sodium oxalate, allows for the highly sensitive detection of 1,3-Dihydroxyacetone. The method does not require any chemical modifications or derivatization of 1,3-Dihydroxyacetone, making it straightforward and highly specific The detection process involves measuring the electrochemiluminescence intensity as a function of the applied potential, which correlates directly with the concentration of 1,3-Dihydroxyacetone present in the sample. The method exhibits two distinct linear responses across the concentration ranges of 5.0-500 μM and 500 μM-6.0 mM, providing flexibility depending on the concentration levels expected in the samples. The sensitivity of this method is particularly notable, with a detection limit as low as 1.79 μM. This level of sensitivity is substantially greater than other techniques used for detecting 1,3-Dihydroxyacetone, often by two orders of magnitude. This makes the electrochemiluminescence method not only innovative but also superior in terms of detection limits, linear range, and usability. In practical applications, the electrochemiluminescence detection of 1,3-Dihydroxyacetone using tris(2,2'-bipyridine)ruthenium(II) can be deployed in quality control processes in the cosmetic and food industries, where accurate quantification of 1,3-Dihydroxyacetone is critical. This method provides a reliable, highly sensitive, and efficient way to monitor 1,3-Dihydroxyacetone levels in various products, ensuring compliance with industry standards and safety regulations. 2
Reference
1. Zeng W, Shan X, Liu L, Zhou J. Efficient 1,3-dihydroxyacetone biosynthesis in Gluconobacter oxydans using metabolic engineering and a fed-batch strategy. Bioresour Bioprocess. 2022; 9(1): 121.
2. Sun J, Gao W, Qi L, et al. Detection of 1,3-dihydroxyacetone by tris(2,2'-bipyridine)ruthenium(II) electrochemiluminescence. Anal Bioanal Chem. 2018; 410(9): 2315-2320.
You may like
Related articles And Qustion
Lastest Price from 1,3-Dihydroxyacetone manufacturers

US $0.00/kg2025-11-28
- CAS:
- 96-26-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000 kg

US $20.00/kg2025-06-06
- CAS:
- 96-26-4
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 10000kg