What is the Production and role of 25-hydroxycholesterol?
Introduction
25-hydroxycholesterol (25-HC; cholest5-en-3β,25-diol) was one of the first commercially available oxysterol. Human tissues contain very low levels of 25-HC, much lower than, for example, 27-HC or 24S-HC. 25-HC is complexed and transported by lipoproteins, and its plasmatic concentration is usually only a few ng/ml.
Production
25-HC can be generated by both enzymatic and nonenzymatic pathways. The enzyme responsible for 25-HC biogenesis is mainly the 25-hydroxylase (CH25H), which uses cholesterol and molecular oxygen as substrates and NADPH as a cofactor. This enzyme is expressed at low levels in several cell types, including hematopoietic cells, epithelial and endothelial cells (ECs), and in macrophages and lymphoid organs. Human atlas also report high expression of CH25H in adipose tissues, lungs, urinary bladder, and gallbladder. In CNS, where cholesterol metabolism is important for brain functioning, Ch25h is expressed by microglial cells, astrocytes, and ECs of the blood-brain barrier (BBB). The expression pattern of CH25H can vary slightly between males and females[1].
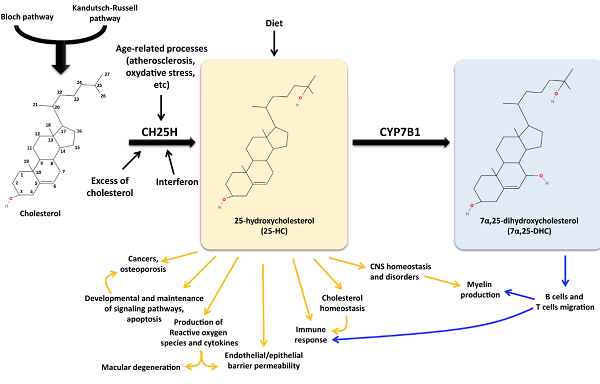
25-hydroxylase (CH25H) converts cholesterol into 25-hydroxycholesterol (25-HC), which is then converted into 7α,25-dihydroxycholesterol (7α,25-DHC) by cytochrome P450 family 7 subfamily B member 1 (CYP7B1). The diet can also provide 25-HC. Many studies report the effects of 25-HC and 7α,25-DHC in several cellular processes, thus underlying their involvement in atherosclerosis, cancer onset and development, immune response, and neurodegenerative disorders.
Interestingly, CH25H expression and 25-HC Production are closely related to the inflammatory and immune situations of the organs or tissues. Ch25h gene has been identified as an interferon-stimulated gene. Its expression is quickly increased in the heart, brain, muscle, kidney, lung, lymphoid organs, and most notably, liver upon in vivo exposure to a toll-like receptor 4 (TLR4) agonist, thus leading to a 25-HC increase in tissues and blood. Similarly, injection of a TLR4 ligand [like lipopolysaccharide (LPS), for example] into human subjects produces a transient increase in serum 25-HC. In addition, in vitro, the Production and secretion of 25-HC are observed in several cell lines, including mouse macrophages, ECs, dendritic cells, astrocytes, and microglial cells treated with inflammatory molecules. Interestingly, it has been demonstrated that CH25H and LXR expressions are controlled by krüppel-like factor 4, a central anti-inflammatory transcription factor, in ECs and macrophages in vitro and in vivo models of atherosclerosis, thus suggesting an atheroprotective role of the krüppel-like factor 4–Ch25h/LXR axis.
Besides, other enzymes belonging to the cytochrome P450s, such as CYP27A1, CYP46A1, and CYP3A4, can also produce small amounts of 25-HC. 25-HC can be further metabolized into 7α,25-dihydroxycholesterol (7α,25-DHC) by the enzyme sterol 7α-hydroxylase (CYP7B1), which also participates in bile acid synthesis. It is important to note that expression patterns of CYP7B1 and CH25H do not fit very well, as observed in many brain regions. Contrary to CH25H, CYP7B1 is highly expressed in the liver and the thyroid.
25-HC can also be 3-sulfated by sulfotransferases such as SULT2B1b to form 25HC3S, which shows altered biological effects compared to 25-HC.
25-HC is one of the most detected oxysterols in case of long storage of cholesterol-rich products. However, it is poorly produced in lipoproteins incubated in oxidative conditions compared with other nonenzymatically produced oxysterols like the 7-ketocholesterol. This indicates that cholesterol autoxidation is of limited importance for forming 25-HC in living organisms, but that 25-HC from dietary intake might be considered in studies using animals or patients.
Role
25-HC was shown to modulate cholesterol metabolism, thus regulating cholesterol biosynthesis. Its ability related to immunity was hinted by the discovery of the enzyme CH25H (cholesterol-25-hydroxylase), which catalyzes the synthesis of 25-HC and was upregulated in immunocytes exposed to inflammatory agents. CH25H was proved to be a member of the interferon-stimulating genes (ISGs) family. Meanwhile, 25-HC seems to have similar features to CH25H and is augmented in macrophages after viral infection and by interferon signaling. It also shows great potential to counteract enveloped viruses[2].
25-HC inhibits the infection of various viruses by modulating sterol synthesis and distribution in cells. Several in vitro studies have shown that 25-HC inhibits infection by multiple enveloped viruses, including Kaposi’s sarcoma herpesvirus, human immunodeficiency virus type 1, Ebola virus, West Nile Virus, Zika virus, etc. In return, some of these viruses can probably downregulate 25-HC production to facilitate their infection process, as demonstrated in endothelial cells infected by Kaposi's sarcoma herpesvirus.
Interestingly, the role of 25-HC in coronavirus disease 2019 infection has been very well documented, and this oxysterol plays a key role in protection against this virus, suggesting that it can be a therapeutic target in future drug development against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Thus, CH25H expression is found to be upregulated in macrophages and lung epithelial cells (EPs) gathered in bronchioalveolar lavage fluid from coronavirus disease 2019 patients, and 25-HC level is elevated in some patients suffering from SARS-CoV-2 infection (84). Recently, it has been demonstrated that 25-HC inhibits RNA-dependent RNA polymerase and the main protease of the SARS-CoV-2 virus, which are both key players in viral transcription and replication.
Among all the existing oxysterols, 25-HC shows a unique synthesis process significantly regulated by inflammation and an exclusive function of reducing HMGCR and LDLR levels via inhibiting the SCAP/SREBP-2 pathway and activating the LXR signaling pathway. These exceptional properties make 25-HC a key player in host defense during viral and bacterial infection. Moreover, 25-HC and its metabolite 7α,25-DHC, have also shown interesting properties by modulating immune cell migration or endothelial/epithelial barrier properties, thus involving them in atherosclerosis and CNS disorders. 25-HC also shows great importance in age-related diseases often associated with altered cholesterol metabolism, oxidative stress, inflammation, and cell death, like AD, atherosclerosis, osteoporosis, or macular degeneration. 25-HC is, therefore, a very promising oxysterol with huge therapeutic potential. Indeed, it might be beneficial to target 25-HC to prevent or dampen the onset and development of these diseases. However, beneficial and deleterious effects are reported for 25-HC in animal and cell models. Therefore, it is unclear if 25-HC levels need to be increased or decreased to treat a disease and whether inflammatory states are necessary.
References:
[1] CINDY NGUYEN. 25-Hydroxycholesterol in health and diseases.[J]. Journal of Lipid Research, 2024. DOI:10.1016/j.jlr.2023.100486.[2] JIALU ZHANG. 25-hydroxycholesterol: an integrator of antiviral ability and signaling.[J]. Frontiers in Immunology, 2023, 14. DOI:10.3389/fimmu.2023.1268104.
You may like
See also
Lastest Price from 25-HYDROXYCHOLESTEROL manufacturers
US $1.50/g2025-09-12
- CAS:
- 2140-46-7
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 100 Tons

US $6.00/kg2025-04-21
- CAS:
- 2140-46-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month