What is the Pharmacokinetics of Dabigatran etexilate mesylate?
Dabigatran etexilate mesylate, also known by its brand name, Pradaxa, is a type of anticoagulant blood thinner that treats and prevents certain types of blood clots in people ages 8 years and older. It's also used to lower the risk of stroke and blood clots in people with atrial fibrillation (AFib). Side effects include stomach upset and bleeding.
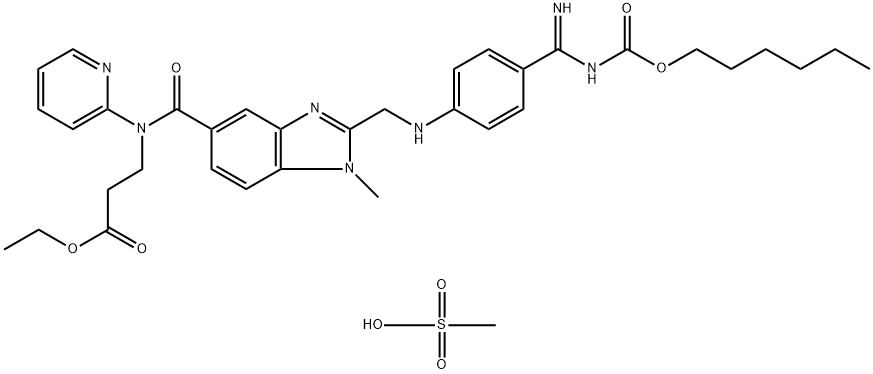
Dabigatran etexilate mesylate is absorbed as the dabigatran etexilate ester. The ester is then hydrolyzed, forming dabigatran, the active moiety. Dabigatran is metabolized to four different acyl glucuronides and both the glucuronides and dabigatran have similar pharmacological activity. Pharmacokinetics described here refer to the sum of dabigatran and its glucuronides. Dabigatran displays dose-proportional pharmacokinetics in healthy subjects and patients in the range of doses from 10 to 400 mg.
Absorption: The absolute bioavailability of dabigatran following oral administration of dabigatran etexilate is approximately 3 to 7%. Dabigatran etexilate is a substrate of the efflux transporter P-gp. After oral administration of dabigatran etexilate in healthy volunteers, Cmax occurs at 1 hour post-administration in the fasted state. Coadministration of PRADAXA with a high-fat meal delays the time to Cmax by approximately 2 hours but has no effect on the bioavailability of dabigatran; PRADAXA may be administered with or without food. The oral bioavailability of dabigatran etexilate increases by 75% when the pellets are taken without the capsule shell compared to the intact capsule formulation. PRADAXA capsules should therefore not be broken, chewed, or opened before administration.
Distribution: Dabigatran is approximately 35% bound to human plasma proteins. The red blood cell to plasma partitioning of dabigatran measured as total radioactivity is less than 0.3. The volume of distribution of dabigatran is 50 to 70 L. Dabigatran pharmacokinetics are dose proportional after single doses of 10 to 400 mg. Given twice daily, dabigatran’s accumulation factor is approximately two.
Elimination: Dabigatran is eliminated primarily in the urine. Renal clearance of dabigatran is 80% of total clearance after intravenous administration. After oral administration of radiolabeled dabigatran, 7% of radioactivity is recovered in urine and 86% in feces. The half-life of dabigatran in healthy subjects is 12 to 17 hours.
Metabolism: After oral administration, dabigatran etexilate is converted to dabigatran. The cleavage of the dabigatran etexilate by esterase-catalyzed hydrolysis to the active principal dabigatran is the predominant metabolic reaction. Dabigatran is not a substrate, inhibitor, or inducer of CYP450 enzymes. Dabigatran is subject to conjugation forming pharmacologically active acyl glucuronides. Four positional isomers, 1-O, 2-O, 3-O, and 4-O-acylglucuronide exist, and each accounts for less than 10% of total dabigatran in plasma.
References
[1] Pradaxa (dabigatran): Uses, Side Effects, Dosage & More - GoodRx https://www.goodrx.com/pradaxa/what-is
[2] PRADAXA (dabigatran etexilate mesylate) capsules for oral use Label https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022512s007lbl.pdf
You may like
Lastest Price from Dabigatran Etexilate Mesylate manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 872728-81-9
- Min. Order:
- 100g
- Purity:
- 98.5%min
- Supply Ability:
- 10KGS

US $6.00/KG2025-03-28
- CAS:
- 872728-81-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20TONS