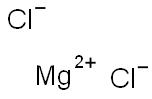What is the charge of magnesium in Magnesium chloride?
Description
Magnesium chloride is a salt that is a typical ionic halide, and they are highly soluble in water. This compound comes in either anhydrous or multiple-hydrated crystal forms. The molecular formula of magnesium chloride is MgCl₂.
Molecular charge
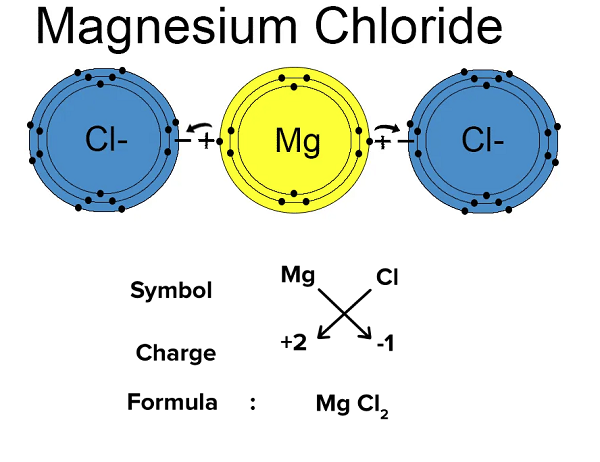
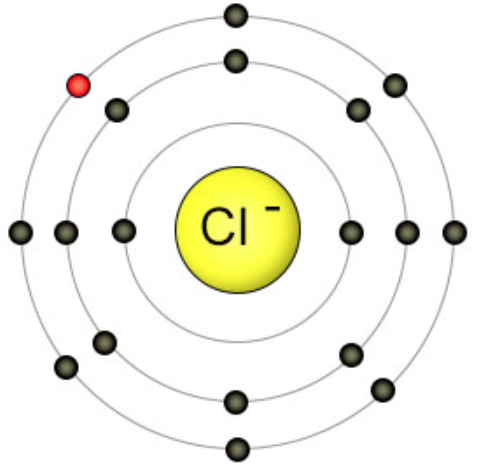
In this molecular, Magnesium chloride is an ionic compound comprising one magnesium and two chlorine atoms. Chloride ion always has a unit negative charge. Since ionic compounds are neutral, the net charge on them is zero.
Charge on magnesium ion + 2×(charge on chloride ion) = 0
Charge on magnesium ion + 2×(-1) = 0
Charge on magnesium ion = +2
Thus, magnesium has two units of positive charge, written as Mg2+.
Uses
Magnesium chloride is one of the naturally occurring inorganic compounds that has a wide variety of applications in industries and medical fields, and it is even an essential mineral for human beings. It is mainly used for dust control, road stabilization, and de-icing of highways and sidewalks in snowy places. Apart from the production of magnesium metal, magnesium chloride is also used for various other applications, including fertilizers and mineral supplements for animals, wastewater treatment, fireproofing agents, types of cement, and refrigeration brine.
You may like
Related articles And Qustion
See also
Lastest Price from Magnesium chloride manufacturers

US $1.00/PCS2025-04-21
- CAS:
- 7786-30-3
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 7786-30-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month