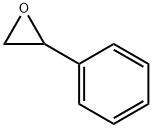
What is Styrene oxide?
Feb 19,2020
Styrene oxide is a fine chemical product, also known as phenylene oxide, or 1,2-epoxyethylbenzene. It is an important intermediate for organic synthesis and is widely used in organic synthesis, pharmaceutical preparation, and perfume production. For example, styrene oxide is added to hydrogen to produce monophenylethanol under the action of a catalyst. It has a wide range of uses, and can be used in floral fragrances for daily use, as well as in food. Styrene oxide is also an important intermediate for the synthesis of levamisole hydrochloride. L-imidazole hydrochloride is a broad-spectrum intestinal repellent that can be used by humans and animals. In recent years, the demand for such fine chemicals at home and abroad has increased dramatically, and styrene oxide has been in short supply. Therefore, no matter in industrial production or organic synthesis, styrene oxide has attracted much attention.
There are various methods for synthesizing styrene oxide. So far, the published literature mostly uses styrene as a raw material to synthesize styrene oxide. Most of the styrene oxide on the market is also prepared by styrene-catalyzed epoxidation. The equation is as follows:
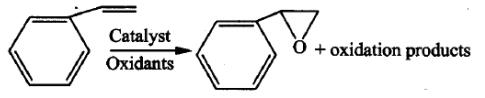
Figure 1. Synthetic reaction equation of styrene oxide
The oxidants of styrene epoxidation are different. The commonly used oxidants are molecular oxygen, hydrogen peroxide (also known as hydrogen peroxide), NaOC1, chloroperoxybenzoic acid (in-CPBA), periodate, tert-butyl Hydrogen peroxide (TBHP) and so on. The epoxidation reaction products of styrene are also very complicated, and different products will be obtained depending on the catalyst used, such as styrene oxide, benzaldehyde, phenylacetaldehyde, and benzoic acid. In recent years, people have mainly studied the styrene epoxidation process from the perspective of oxygen sources and catalysts. The current research hotspot is to use economical and environmentally friendly oxidants (such as oxygen and hydrogen peroxide) with high-performance catalysts to achieve industrial production with zero pollution to the environment.
In addition to the catalytic oxidation reaction of styrene to produce styrene oxide, it can also be prepared by addition and elimination reactions. For example, styrene and hypohalous acid can undergo an addition reaction to produce 2-halophenylethanol, and then undergo an elimination reaction in the presence of a base to produce styrene oxide. This is the classic method for preparing styrene oxide. For example, bromophenyl alcohol method, chlorophenyl alcohol method, etc., all of them can obtain good yields.
References
[1] Zolezzi S, Spodine E, Decinti A. Epoxidation of styrene with iodosylbenzene in the presence of copper(II)Sehifbase complexes[J]. Polyhedron, 2003, 22(13): 1653
—1658.
[2] PatilN s, Uphade B s, Jana P, et a1. Epoxidation of styrene by anhydrous t-butyl hydroperoxide over reusable gold supported on MsO and other alkaline earth oxides[J]. J Catal, 2004, 223: 236-239.
[3] DING Li-qin, ZHANG Jun-tao, LIANG Sheng-rong, WANG Xiao-quan. Research progress in the catalysts for the preparation of styrene oxide by the epoxidation of styrene[J]. Journal of Xian Shiyou University, 2011, 4: 71-77.
You may like
Ritalinic Acid: a Methylphenidate Metabolite
Dec 26, 2025
See also
Lastest Price from Styrene oxide manufacturers
Styrene oxide

US $0.00/kg2025-04-15
- CAS:
- 96-09-3
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons
Styrene Oxide

US $10.00/KG2022-06-02
- CAS:
- 96-09-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 tons