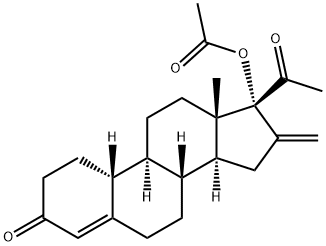
What is Segesterone acetate (Nestoron)?
General description
Segesterone acetate (SA; Nestoron) is a potent non-androgenic progestin that was developed for contraception and it is the progestin in the once-yearly contraceptive vaginal ring[1]. SA has an orthorhombic crystalline structure with space group P212121 and the chemical formula C24H30O4. In 2018, it was approved as a combination therapy with ethinyl estradiol, marketed under the brand name Annovera. This drug represents the first in a new class of contraceptives, as it is administered as a silicone ring that can be inserted by the patient, does not require refrigeration, and whose duration of action lasts for an entire year. These factors are all especially beneficial for providing effective contraception in limited-resource settings and developing nations.
Synthesis method
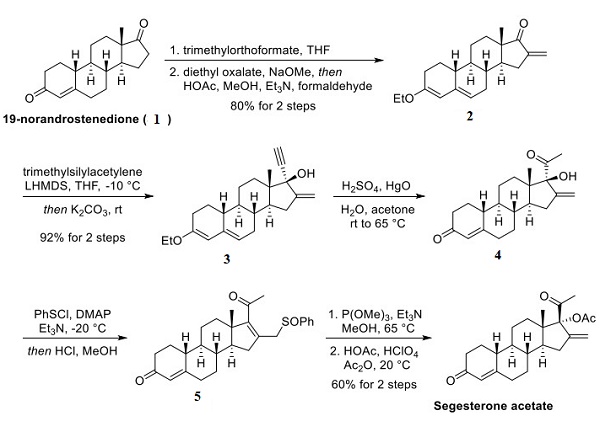
Although detailed synthetic routes to segesterone acetate are sparsely available, a 1997 paper from The Population Council cited earlier work by Mehrof et al. and Schwars et al. for a general synthetic approach to the compound. A 2013 patent by the same group disclosed an updated and concise synthesis involving the nucleophilic addition of an acetylene equivalent into a ketone, followed by hydration to obtain the desired functionality. The route began with 19- norandrostenedione (shown below)[2]. In a two-step, one-pot procedure, the enone was converted to the enol ether using trimethyl orthoformate, followed by formylation of the remaining ketone to provide compound 2 in an 80% yield over two steps. Next, trimethylsilyl acetylene was deprotonated using LiHMDS in THF prior to subjection to compound 2. After the addition was complete, the reaction was allowed to warm to room temperature, and the TMS group was removed with potassium carbonate to generate compound 3. The alkyne was then hydrated under acidic conditions to the corresponding ketone 4. The next series of reactions enabled the inversion of configuration at the congested C-17 center. Treatment of alcohol 4 with phenylsulfuryl chloride resulted in chloride displacement and a 2,3-sigmatropic rearrangement to arrive at sulfoxide 5. The subjection of the enone of compound 5 to trimethoxyphosphine and triethylamine, followed by treatment with hypochlorous acetic anhydride conditions, induced a Mislow−Evans rearrangement to complete the preparation of segesterone acetate.
References
[1] Fermin F.H. Aragon . “Polymorphism characterization of segesterone acetate: A comprehensive study using XRPD, FT-IR and Raman spectroscopy.” International Journal of Pharmaceutics 596 (2021): Article 120234.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
See also
Lastest Price from Nestoron manufacturers

US $0.00/GRAM2025-08-26
- CAS:
- 7759-35-5
- Min. Order:
- 100GRAM
- Purity:
- 99%
- Supply Ability:
- 500KGS