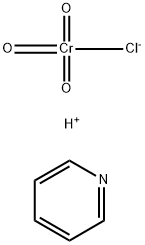
What is Pyridinium chlorochromate(PCC)?
Identification
Product Name: Pyridinium chlorochromate
Synonyms: PCC;PYRIDINIUM CHLOROCHROMATE;chromate(1-),chlorotrioxo-,(beta-4)-,hydrogen,compd.withpyridine(1:1);Pyridine, (T-4)-chlorotrioxochromate(1-);Pyridine, chlorotrioxochromate(1-);Urine Luck;pyridinium;PYRIDINIUM CHLOROCHROMATE, CA. 20 WT. % ON BASIC ALUMINA
CAS: 26299-14-9
MF: C5H6ClCrNO3
MW: 215.56
EINECS: 247-595-5
Properties
Melting point 205-208 °C(lit.)
Storage temp. 2-8°C
Solubility Soluble in acetone, benzene, dichloromethane, acetonitrile and tetrahydrofuran.
Form Crystalline Powder
Color Orange
Sensitive Moisture Sensitive
Merck 14,7974
BRN 7054643
Stability: Stable. May react with easily oxidized materials. Incompatible with reducing agents, combustible material, metals, strong reducing agents.
Used as a Reagent
Pyridinium chlorochromate is usually used to oxidize an allylic methylene group. Pyridinium dichromate (PDC) or pyridinium chlorochromate (PCC) in anhydrous media such as dichloromethane oxidizes primary alcohols to aldehydes while avoiding overoxidation to carboxylic acids.
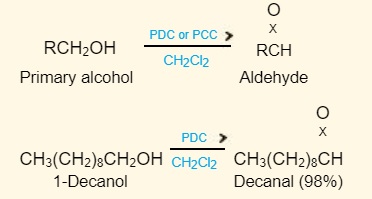
Oxidation of primary alcohols to aldehydes
Pyridinium-chlorochromate is a stable versatile oxidizing agent for mild and selective oxidation of alcohols. Used to oxidize an allylic methylene group in a synthesis of α,ß-unsaturated lactones from D-glucose using Grubbs-catalyzed ring-closing metathesis.
Pyridinium chlorochromate is used as an oxidizing agent to convert primary and secondary alcohols to aldehydes and ketones respectively. It is involved in the preparation of cyclohexanone, (-)-pulegone and lactones. It plays an important role in the selective mono-oxidation of xylenes to tolualdehydes and arylhydroxyamines to nitroso compounds. Furthermore, it is utilized as an oxidant for amino acids, L-cystine, aniline, cycloalkanols, vicinal and non-vicinal diols as well as in the Babler oxidation reaction.
Reactions
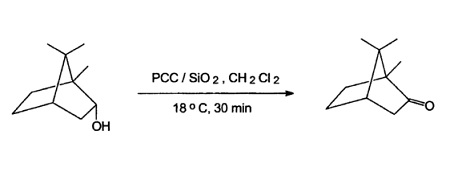
Pyridinium chlorochromate (PCC) is a widespread used oxidation reagent but lacks of selectivity with unsaturated compounds. Furthermore, the workup procedure is quite tedious. Absorption of PCC onto silica gel improves the oxidation but then a reagent excess is necessary to reach appropriate rates. These drawbacks can be overcome by sonication leading to short reaction times and only an small excess of 1.2.-1.5 eq. of PCC is required. Using this reagent has been used to oxidise borneol to camphor in 85% yield.
You may like
Lastest Price from Pyridinium chlorochromate manufacturers

US $0.00/kg2025-04-11
- CAS:
- 26299-14-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons
US $85.00/Kg2024-10-06
- CAS:
- 26299-14-9
- Min. Order:
- 0.100Kg
- Purity:
- 98
- Supply Ability:
- 1000 Kg