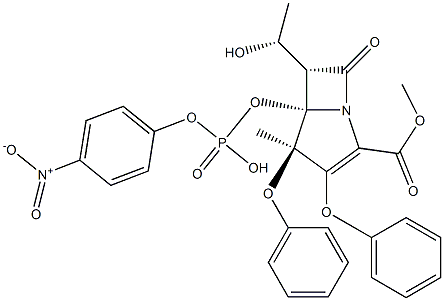
What is Proteinase K?
Description
Proteinase K is a medium-sized globular α/β protein (279 amino acids) with an available high-resolution X-ray crystal structure. This substance is a subtilisin-like serine protease from Tritirachium album limber with a broad-spectrum degradation capability to degrade proteins. Proteinase K is commonly used in molecular biology to digest protein and remove contamination from preparations of nucleic acid. The addition of Proteinase K to nucleic acid preparations rapidly inactivates nucleases that might otherwise degrade the DNA or RNA during purification.
Property
Activated by calcium, the enzyme digests proteins preferentially after hydrophobic amino acids (aliphatic, aromatic, and other hydrophobic amino acids). Although calcium ions do not affect the enzyme activity, they do contribute to its stability. Proteins will be completely digested if the incubation time is long and the protease concentration is high enough. Upon removal of the calcium ions, the stability of the enzyme is reduced, but the proteolytic activity remains. Proteinase K has two binding sites for Ca2+, which are located close to the active center but are not directly involved in the catalytic mechanism. The residual activity is sufficient to digest proteins, which usually contaminate nucleic acid preparations. Therefore, the digestion with Proteinase K for the purification of nucleic acids is usually performed in the presence of EDTA (inhibition of metal-ion dependent enzymes such as nucleases). Research has found that proteinase K has lower thermostability (or structural stability) at high solvent temperatures than at low solvent temperatures[1].
Uses
Proteinase K (EC 3.4.21.64) is widely used in molecular biology because of its broad substrate specificity for hydrolysis of esters as well as its ability to cleave the peptide bond proximate to the carboxyl group of aliphatic and aromatic amino acids. In addition, proteinase K is active at a broad pH range (7.5-12.0), under which conditions most other proteases cannot function[2]. These characteristics make proteinase K applicable to chemoenzymatic peptide synthesis, a clean, mild, atom-economical, and stereoselective method of forming peptide bonds via aminolysis. However, the thermal stability of proteinase K limits its practical potential as an efficient catalyst.
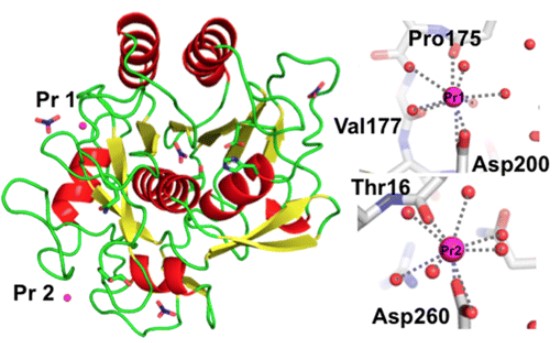
To solve this limitation, Yazawa enhanced the thermal stability of proteinase K using a heavy-atom derivatization technique. Since proteinase K usually requires two Ca ions for activation of enzymatic catalysis and thermal stability. Removal of Ca ions is known to trigger a concerted domino-like conformational change in proteinase K, which decreases its enzymatic activity to 20% of its original value. They developed a simple technique that involves the incubation of proteinase K with heavy atoms, such as praseodymium (Pr), to stabilize proteinase K. As a result, they found that Pr ions increased both hydrolytic and aminolysis activities of proteinase K. Pr-derivatized proteinase K showed 3.9-fold higher hydrolytic activity and 9.5-fold higher aminolysis activity at 60 °C, compared with Ca-binding proteinase K. Binding of ligands to enzymes is well-known to enhance their thermal stability[3].
References
[1] Song P, et al. Effect of the Solvent Temperatures on Dynamics of Serine Protease Proteinase K. International Journal of Molecular Sciences, 2016; 17: 254.
[2] Breibeck J, et al. Speciation of Transition-Metal-Substituted Keggin-Type Silicotungstates Affected by the Co-crystallization Conditions with Proteinase K. Inorganic Chemistry, 2021; 60: 15096–15100.
[3] Yazawa K, et al. Derivatization of Proteinase K with Heavy Atoms Enhances Its Thermal Stability. ACS Catalysis, 2016; 6: 3036–3046.
You may like
Related articles And Qustion
See also
Lastest Price from Proteinase K manufacturers

US $0.00/g2025-04-21
- CAS:
- 39450-01-6
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 50kg/month

US $6.00/kg2025-04-21
- CAS:
- 39450-01-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month