What is Oxalyl chloride?
Description
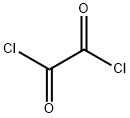
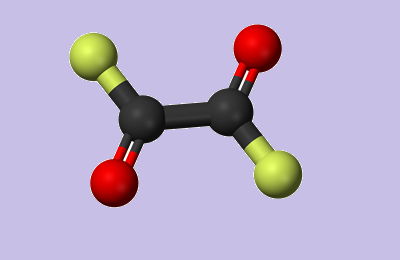
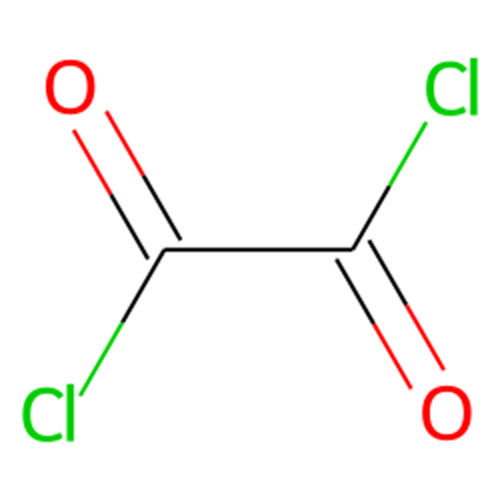
Oxalyl chloride, also known as oxalyl dichloride, is a highly toxic chemical with the formula (COCl)2. It is a colorless, strong-smelling liquid belonging to the diacetyl chloride group; Soluble in benzene, hexane, ether, and halogenated solvents such as chloroform, acetonitrile, and dichloromethane[1]. Oxaloyl chloride reacts with water to release toxic gases: hydrogen chloride and carbon monoxide.
Synthesis
In 1892, French chemist Adrien Fauconnier first prepared oxalyl chloride by the reaction of diethyl oxalate with phosphorus pentachloride (PCl5). [2] Oxalyl chloride can also be prepared by treating oxalic acid with PCl5, and the reaction is as follows:
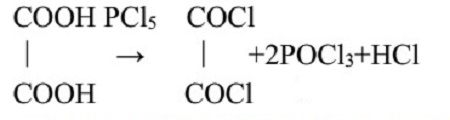
Uses
Oxaloyl chloride has two chlorines that are easy to eliminate and have high reactivity, so it has become one of the most active organic acid chlorides and organic reagents and is widely used as an important intermediate to synthesize various chlorinated compounds. Oxaloyl chloride can be used as a reagent in chlorination, oxidation, reduction, dehydration, decarboxylation, and formylation reactions[2]. The discovery of oxaloyl chloride/DMSO/alkali systems led to the development of the oxidation of alcohols to aldehydes and ketones, showing particularly important achievements in particular oxidation known as Swern oxidation. Swern oxidation is one of the named reactions in organic chemistry that uses oxalyl chloride to convert alcohols to aldehydes and ketones. Oxalyl chloride of acid or alcohol functional group activation, so that it can be converted, such as esterification, amidation, and intramolecular cyclization reactions[3].
The highly toxic nature of chlorine oxalate cannot be ignored in use and must be used at low temperatures in some reactions. In order to avoid adverse side effects, oxaloyl chloride should be used in stoichiometry in experiments. Zhang et al. developed a fluorescent sensor that can selectively check oxalyl chloride in solution with a detection limit as low as 3 nM[4].
References
[1] Mohammadkhani L, et al. Oxalyl Chloride: A Versatile Reagent in Organic Transformations. ChemistrySelect , 2019; 4: 6309-6337.
[2] Gao Y, et al. Dimethyl sulfoxide/oxalyl chloride: A useful reagent for sulfenyletherification. Synthetic Communications , 2018; 48: 2773-2781.
[3] Ding R, et al. Dichlorination of olefins with diphenyl sulfoxide/oxalyl chloride. Synthetic Communications , 2020; 50: 2319-2330.
[4] Zhang W, et al. A benzothiadiazole-based fluorescent sensor for selective detection of oxalyl chloride and phosgene. Organic Chemistry Frontiers, 2017; 4: 1719-1725.
You may like
Related articles And Qustion
See also
Lastest Price from Oxalyl chloride manufacturers

US $1.00/KG2025-05-14
- CAS:
- 79-37-8
- Min. Order:
- 1000KG
- Purity:
- 99%
- Supply Ability:
- Q: 20000 MT

US $1.00/PCS2025-04-21
- CAS:
- 79-37-8
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 100mt