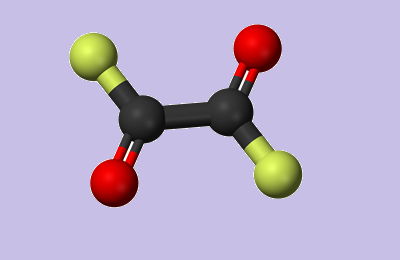
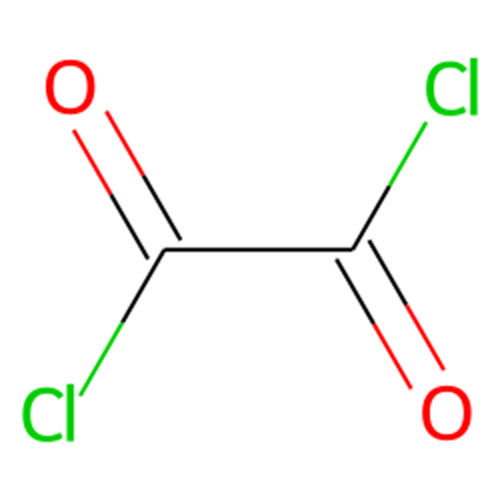
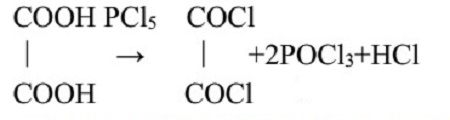The Synthesis of Oxalyl Chloride
Oxalyl chloride, the most active of the organic acid chlorides, approaches in activity as a chlorinating or dehydrating agent, inorganic chlorides such as thionyl chloride or phosphorus trichloride. It is more versatile as a reagent than alternative organic acid chlorides. As a bifunctional acid chloride, oxalyl chloride reacts with diamines to form polyamides. It even reacts with both of the nitrogens of urea to form oxalyl urea.

Synthesis
The oxalic acid must be dry or the yield of oxalyl chloride is cut down to a minimum. Drying may be achieved in an oven at 110℃ for 6-8 hours, having the oxalic acid spread out in a thin layer.During the drying, the oxalic acid tends to cake and thus must be broken up in a mortar from time to time.
To round bottom flask which is cooled with ice-water bath 90g anhydrous powdered oxalic acid is mixed with 400g of pulverized phosphorus pentachloride. The mixture is allowed to stand for 2-3 days at room temperature until the mass is fully liquefied. The reaction products are fractionally distilled by collecting fraction between 60 and 100℃. By repeated distillation, oxalyl chloride is obtained entirely free from phosphorus oxychloride. The yield of the final product is about 50% which is a colorless liquid, with boiling 64℃.
You may like
Related articles And Qustion
Lastest Price from Oxalyl chloride manufacturers

US $1.00/KG2025-05-14
- CAS:
- 79-37-8
- Min. Order:
- 1000KG
- Purity:
- 99%
- Supply Ability:
- Q: 20000 MT

US $1.00/PCS2025-04-21
- CAS:
- 79-37-8
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 100mt