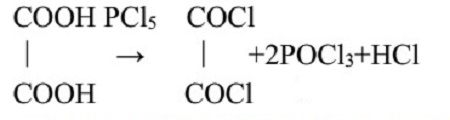Reactions and Applications of Oxalyl Chloride
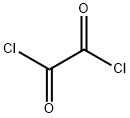
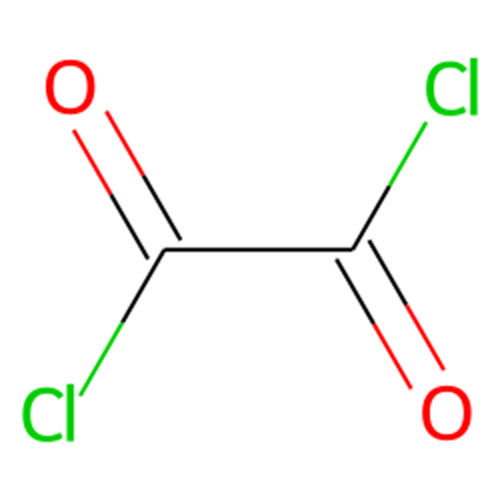
Oxalyl chloride
Oxalyl chloride has found general application for the
preparation of carboxylic acid chlorides since the reagent was introduced by Adams and Ulich. Acid chlorides produced by this
means have subsequently featured in the synthesis of acyl azides,
bromoalkenes, carboxamides, cinnolines, diazo ketones,
(thio)esters, lactones, ketenes for cycloaddition reactions,
intramolecular Friedel–Crafts acylation reactions, and the
synthesis of pyridyl thioethers.
Like Thionyl Chloride, oxalyl chloride gives gaseous byproducts with acids and the chlorides can be readily isolated in a pure form by evaporation of the solvent and any excess reagent, or used in situ for further elaboration (eq 1).
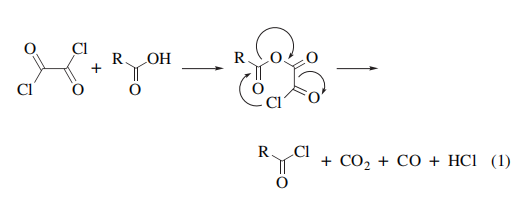
Prior formation of an amine or alkali metal salt, with or without pyridine, has been used to advantage with substrates that are sensitive to strong acids or are bases (see also Oxalyl Chloride–
Dimethylformamide for a procedure conducted under neutral
conditions using silyl esters). By adjusting the molar proportions
of oxalyl chloride to substrate, anhydrides can also be prepared
using these methods (eq 2). N-Carboxy-α-amino acid anhydrides can also be made this way.
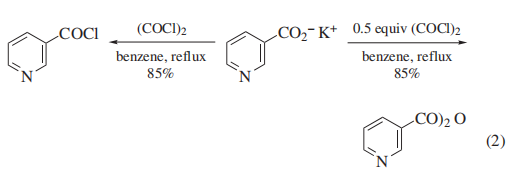
The use of nonpolar solvents such as hexane or toluene allows
for the removal of inorganic or amine salts which may otherwise
interfere with subsequent reactions. Under the mild conditions employed (eqs 3 and 4), racemization of stereogenic centers, skeletal rearrangement, or byproduct formation, seen with other reagents such as thionyl chloride/
pyridine, are seldom observed.
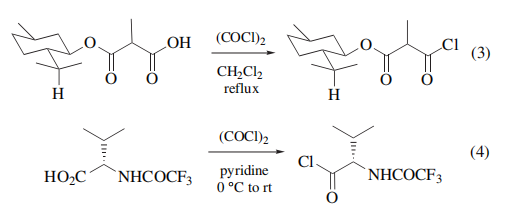
Conversion of β-bromoacrylic acid to the acid chloride
using thionyl chloride/DMF, Phosphorus(III) Chloride, or benzotrichloride/zinc chloride also resulted in bromine for chlorine
exchange. Use of oxalyl chloride with the preformed ammonium
salt provided a mild, general method to β-bromoacryloyl chlorides (eq 5)19 without halogen exchange or (E/Z) equilibration.
β-Fluoro- and iodoacrylic acids have been cleanly converted to
the acid chlorides without prior salt formation.
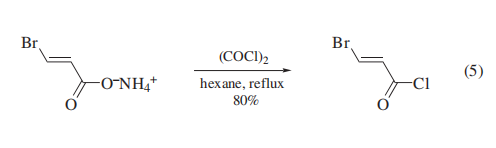
Formation of Chloroiminium Salts
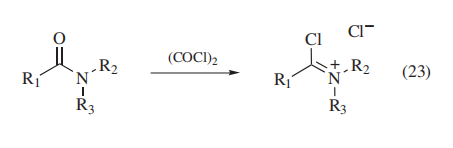
Oxalyl chloride reacts
readily with amides or lactams to afford chloroiminium salts
that have many synthetic applications (eq 23).
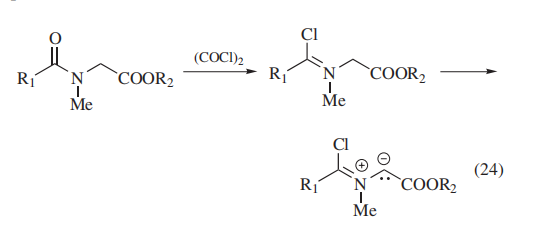
For example, efficient syntheses of thioamides and thiolactams are based on this methodology. Certain types of chloroiminium salts can serve as precursors for high-energy synthetic intermediates. Thus, azomethine ylides were obtained by treatment of β-acylamino carboxylic esters with oxalyl chloride (eq 24).
References:
[1] BONCLI Y. oxalyl chloride[J]. Catalysis from A to Z, 2020, 37 1. DOI:10.1002/9783527809080.cataz12065.
Related articles And Qustion
See also
Lastest Price from Oxalyl chloride manufacturers

US $1.00/KG2025-05-14
- CAS:
- 79-37-8
- Min. Order:
- 1000KG
- Purity:
- 99%
- Supply Ability:
- Q: 20000 MT

US $1.00/PCS2025-04-21
- CAS:
- 79-37-8
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 100mt