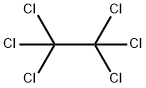
What is Hexachloroethane?
Description
Hexachloroethane is a member of the class of chloroethanes, which is ethane, in which all the hydrogens are replaced by chloro groups. It is also called HCE, perchloroethane, and carbon hexachloride. It has a role as a carcinogenic agent and a refrigerant.
Chemical property
Hexachloroethane (HCE) is a halogenated hydrocarbon consisting of six chlorines attached to an ethane; it is a white to pale yellow solid that is unstable in air and evaporates gradually. It smells like camphor when its concentration in air and water are 150 and 10 ppb, respectively. HCE itself does not catch fire quickly; however, in aqueous non-biological conditions, it has been determined that HCE is unstable, and non-enzymatic dechlorination in the absence of nicotinamide adenine dinucleotide phosphate (NADP) occurs. It rapidly degrades in soil or groundwater. Also, some microorganisms break down HCE without oxygen, and decomposition in aerobic conditions has been reported. Some bioconcentration of HCE in fish has been determined, though upper levels through the food chain are limited since it is rapidly metabolized by fish, which is discussed later.
Uses

In the United States, the military uses about half of the hexachloroethane for smoke-producing devices. Another use of hexachloroethane is in pyrotechnics. It inhibits the explosiveness of methane and the combustion of ammonium perchlorate. Hexachloroethane is used in sheep and cattle as an anthelmintic (to destroy tapeworms). It is also added to the feed of ruminants to prevent methanogenesis and increase feed efficiency, and it is used as an ingredient in some fungicides and insecticides. Hexachloroethane is used in metal and alloy production. Hexachloroethane has various applications as a polymer additive. It has flameproofing qualities and increases affinity for dyes.
Toxicity
Eyes, skin, respiratory system, CNS, liver and kidneys have been proposed as main targets in humans upon exposure. Symptoms include blinking, tearing, photophobia, and irritation of the eyes. Also, facial muscles may have difficulty in movement. Animal studies on the effects of HCE during pregnancy are limited but show effects only at maternally toxic doses. After oral exposure, HCE is primarily distributed to fat tissue. Toxicokinetic studies in animals indicated that HCE is mostly localized and metabolized in the liver and kidney. Several corresponding metabolites have demonstrated liver and kidney toxicities similar to HCE. Neurological effects such as tremors and ataxia were observed in Beagle dogs and pregnant rats. Other effects via inhalation exposure included reduced body weight and increased relative liver weight in rats and guinea pigs. Based on California Proposition 65, HCE was proposed to be carcinogenic for humans, and it induces tumours at sites other than the site of entry. Noncancerous effects include kidney degeneration (tubular nephropathy, necrosis of renal tubular epithelium, hyaline droplet formation, tubular regeneration, and tubular casts) and hepatocellular necrosis. It results in hyaline droplet nephropathy and renal toxicity. It induces chromosome malsegregation, lethality, and mitotic growth arrest, but altogether, from the results of the majority of genotoxicity studies, the genotoxic potential can be evaluated as low.
Lastest Price from Hexachloroethane manufacturers

US $9.90/KG2025-04-21
- CAS:
- 67-72-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $0.00/KG2025-03-05
- CAS:
- 67-72-1
- Min. Order:
- 1KG
- Purity:
- >99%
- Supply Ability:
- 100 MT