Thioanisole: Chemical Properties, Applications and Photodissociation
General Description
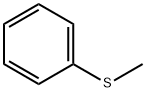
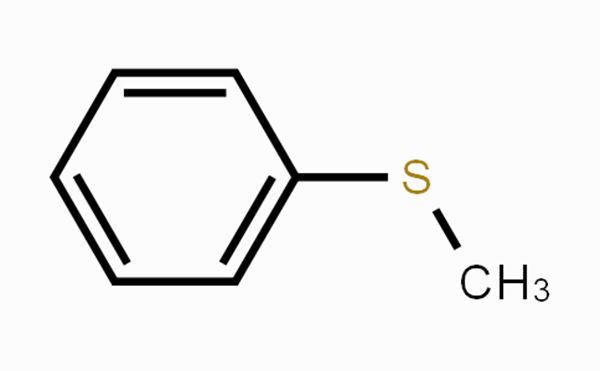
Thioanisole is an aromatic sulfide. It is a colorless to pale yellow transparent liquid with an unpleasant odor. It is insoluble in water and soluble in organic solvents such as ether, benzene, alcohol and oil. It is used as a flavoring agent in the food field. It can also be used to synthesize other organic reagents such as methyl phenyl sulfoxide by oxidation.

Figure 1. Thioanisole
Applications
Thioanisole is a commonly used scavenger in peptide synthesis. It can intercept the released nucleophilic substances in the global deprotection step of peptide side chains, which is common in the cleavage process of benzyloxycarbonyl, Tosyl, Mts and Mtr. However, during the acid-mediated global deprotection of peptide side chains, Thioanisole can induce various uncontrolled modifications of sensitive amino acids (such as Met, Trp, Glu or Asp), especially in the presence of high concentrations of strong acids (such as TFMSA or HF). Therefore, it is best to avoid using it when performing global deprotection of side chains of peptides containing Trp and Met under the guidance of HF or TFMSA to prevent unwanted alkylation side reactions.
In addition, the tosyl group attached at the guanidino function of arginine can be efficiently cleaved by a thioanisole–trifluoromethanesulphonic acid system, which can deprotect O-2,6-dichlorobenzyltyrosine without the formation of O-to-C rearrangement products; the NG-mesitylene-2-sulphonyl group was also cleaved by a thioanisole–trifluoroacetic acid system.
Comparative study of sulfoxidation activity of free and immobilized Rhodococcus rhodochrous IEGM 66 cells was performed. Free Rhodococcus cells (in the presence of 0.1 vol % n-hexadecane) displayed maximal oxidative activity towards thioanisole (0.5 g/l), a prochiral organic sulfide, added after 48-h cultivation of bacterial cells. Higher sulfide concentrations inhibited sulfoxidation activity of Rhodococcus. Use of immobilized cells allowed the 2-day preparatory stage to be omitted and a complete thioanisole bioconversion to be achieved in 24 h in the case that biocatalyst and 0.5 g/l thioanisole were added simultaneously. The biocatalyst immobilized on gel provides for complete thioanisole transformation into (S)-thioanisole sulfoxide (optical purity of 82.1%) at high (1.0-1.5 g/l) concentrations of sulfide substrate.
Photodissociation
A mode-specific effect was found in the photodissociation studies of Thioanisolez, whereby excitation of the S–CH3 stretching mode leads to trajectories closer to the S1-S2 cone intersection, whereas excitation of other modes with similar excitation energies does not produce this effect. Furthermore, despite similar steady-state rate constants, excitation of the S–CH3 stretching mode leads to faster dissociation times. However, there is no significant difference in the distribution of translational energies upon dissociation, so the simulations do not reproduce the experimental “resonance” of the product branching ratios. The simulated translational energy distribution is lower than the experimental one, implying that during the dissociation process, an excessive amount of available energy is converted into vibrational energy. It will be interesting to see whether different results would be produced if the nuclear motion is treated quantum mechanically rather than classically. While the simulations cannot directly explain the different final energy distributions observed experimentally, the significant changes in the minimum gap distribution and dissociation lifetime do explain why the experimental results differ upon excitation of the S-CH3 vibrational stretching mode.
References:
[1] YANYANG LIU. Selective Photocatalytic Oxidation of Thioanisole on DUT-67(Zr) Mediated by Surface Coordination[J]. Langmuir, 2020, 36 9: 2155-2492. DOI:10.1021/acs.langmuir.9b02582.[2] SHAOHONG L LI D G T. Full-dimensional multi-state simulation of the photodissociation of thioanisole.[J]. Journal of Chemical Physics, 2017, 147 4. DOI:10.1063/1.4994923.
You may like
Related articles And Qustion
Lastest Price from Thioanisole manufacturers

US $10.00/KG2025-04-21
- CAS:
- 100-68-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $79.00-38.00/kg2025-04-21
- CAS:
- 100-68-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton