ChemicalBook > Articles Catagory List >Catalyst-and-Auxiliary >what-is-di-chlorobis-1-2-3-1-phenyl-2-propenyl-dipalladium-ii-
What is di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(ii)?
Feb 12,2020
Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(ii) is an important organic regant for the use of transition-metal-mediated organic syntheses. Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(ii) can be used as a catalyst for the ammonia cross-coupling reactions to synthesize arylamines and conversion of aryl triflates to fluorides.
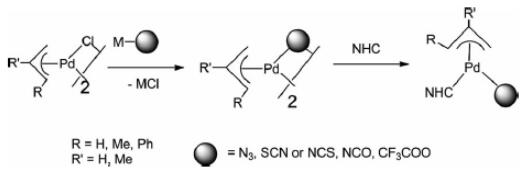
In addition, di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(ii) is an important organic intermediate to synthesize (π-Allyl)Pd complexes containing N-heterocyclic carbene and pseudohalogen ligands. In transition-metal-mediated organic syntheses, Pd-catalyzed C–C cross-coupling reactions are widely utilized. In particular, palladium compounds containing labile ligands such as C,N-donor ligands have emerged as efficient precatalysts for Suzuki–Miyaura cross-coupling reactions. Furthermore, to enhance catalytic efficiency in the catalytic reactions, several studies on cyclopalladated compounds containing N-heterocyclic carbenes (NHCs), one of the many sterically and/or electronically beneficial ligands, were used. Pseudohalogen ligands are known to be better leaving groups than halogen ligands in catalytic reactions. Their catalytic activity was dependent on the supporting ligand, a tertiary phosphane or an NHC ligand. For example, compounds with an NHC ligand showed higher catalytic activity for aryl chlorides than those with a combinationof C,N-donor and tertiary phosphane ligands [1].
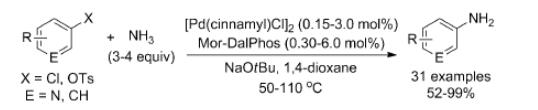
In using [{Pd (cinnamyl)Cl}2]/Mor-DalPhos precatalyst mixtures, electron-rich, electron-poor, and heterocyclic products of ammonia monoarylation were prepared (50–110OC) in high isolated yields. Mor-DalPhos has also been found to be useful in facilitating the Pd-catalyzed monoarylation of hydrazine, acetone, and other carbonyl compounds in chemoselective and aqueous BHA chemistry, as well as in the Au-catalyzed hydroamination of internal alkynes with dialkylamines. Whereas the room-temperature BHA of aryl chlorides with ammonia was not successful when using [{Pd (cinnamyl)Cl}2]/Mor-DalPhos precatalyst mixtures, unprecedented room-temperature transformations of a small number of aryl chlorides were achieved with good monoarylation selectivity by use of the chlorobenzene oxidative addition product [(k2-P,N-Mor-Dal-Phos)Pd(Ph)Cl] as a precatalyst. It is possible to overcome the limitations of other catalysts which include substrate-scope challenges with respect to the monoarylation of electronically deactivated and sterically unhindered aryl chloride substrates, the frequent requirement of relatively high reaction temperatures.[2]
References
1.Kim HK et al. (π-Allyl)Pd Complexes Containing N-Heterocyclic Carbene and Pseudohalogen Ligands – Synthesis, Reactivity toward Organic Isothiocyanates and Isocyanides, and Their Catalytic Activity in Suzuki–Miyaura Cross-Couplings[J]. Eur. J. Inorg. Chem. 2013, 4958–4969
2. Alsabeh PG et al. An Examination of the Palladium/Mor-DalPhos Catalyst System in the Context of Selective Ammonia Monoarylation at Room Temperature[J]. Chem. Eur. J. 2013, 19, 2131 – 2141.
Related articles And Qustion
See also
Formic Acid
Apr 30, 2019
Lastest Price from Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(II) manufacturers
Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(II)
![12131-44-1 Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(II)](/ProductImageEN1/2025-06/Small/650a5153-7cd4-4b6c-b3e6-9b13809b9c61.png)
US $2.00-5.00/kg2025-06-17
- CAS:
- 12131-44-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg
Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(II)
![12131-44-1 Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(II)](/ProductImageEN/2023-01/Small/e5013789-c6e1-45e5-9ca4-f703de0175e8.jpg)
US $0.00/KG2025-04-21
- CAS:
- 12131-44-1
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
![12131-44-1 di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(ii); catalyst; reaction; application](https://www.chemicalbook.com/CAS/20211123/GIF/12131-44-1.gif)