ChemicalBook > Articles Catagory List >Amino-Acids-and-Derivatives >what-is-cis-4-hydroxy-d-proline-hydrochloride-
What is cis-4-Hydroxy-D-proline hydrochloride?
Feb 19,2020
Cis-4-Hydroxy-D-proline belongs to the class of organic compounds known as proline and derivatives. Proline and derivatives are compounds containing proline or a derivative thereof resulting from a reaction of proline at the amino group or the carboxyl group, or from the replacement of any hydrogen of glycine by a heteroatom. cis-4-Hydroxy-D-proline is a substrate that may be used to study the specificity and kinetics of D-alanine dehydrogenase.D-proline in which a hydrogen at the 4-position of the pyrrolidine ring is substituted by a hydroxy group (R-configuration).
The starting point was trans-4-hydroxy-L-proline (10). This was converted (Scheme 3) by a literature method11 into cis-4-hydroxy-D-proline hydrochloride (11) which was esterified in a standard way[1]. A mixture of acetic anhydride (204 g, 2 mol) and glacial acetic acid (600 mL, 10.5 mol) was heated to 50°C at which point (2S,4R)-4-hydroxyproline (Hyp) (47.09 g, 360mmol) was added in one portion. The mixture was refluxed for 5.5 h. After cooling to rt, the solvent was removed under reduced pressure. The residue was dissolved in 2 M HCl (650 mL) and refluxed for another 3h. After cooling to rt the mixture was filtered through Celite and concentrated by rotary evaporation until white needles were formed. The precipitate was collected by suction filtration, washed with Et2O and dried in vacuo to leave pure (2R,4R)-4-hydroxypyrrolidine-2-carboxylic acid hydrochloride[2].
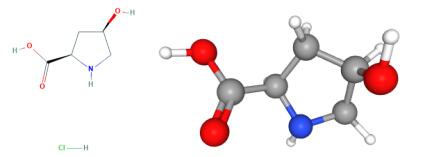
Fig 1. Chemical structure formula and three-dimensional structure of cis-4-Hydroxy-D-proline hydrochloride
Cis-4-Hydroxy-D-proline may be used as a starting material for the 13-step synthesis of new conformationally restricted PNA adenine monomer and the synthesis of N-Benzyl pyrrolidinyl sordaricin derivatives. Cis-4-Hydroxy-D-proline is a substrate that may be used to study the specificity and kinetics of D-alanine dehydrogenase. Cis-4-Hydroxy-D-proline may be used to analyze the substrate specificity of amino acid transporter PAT1.
Epimerization of trans-4-hydroxy-l-proline to cis-4-hydroxy-d-proline during acid hydrolysis of collagen:
Trans-4-Hydroxy-l-proline is epimerized to cis-4-hydroxy-d-proline under the conditions generally used for the hydrolysis of proteins for analyses of amino acids.The cis epimer elutes from a column of UR-30 resin on a Beckman amino acid analyzer at the same time as threonine and hence would be undetected.About 8% of the trans-4-hydroxy-l-proline in collagen can be epimerized to cis-4-hydroxy-d-proline in the hydrolysis of collagen with 6 N HCl at 110℃for 72 hr.
The hypo gene from Sinorhizobium meliloti, located within the trans-4-hydroxy-L-proline metabolic gene cluster, was first successfully expressed in the host Pseudomonas putida. Purified HypO protein functioned as a FAD-containing cis-4-hydroxy-D-proline dehydrogenase with a homomeric structure[3].
References
[1]Baker, G. L.; Fritschel, S. J.; Stille, J. R.; Stille, J. K. J. Org. Chem.1981, 46, 2954–2960.
[2]Heindl, C.; Hu¨bner, H.; Gmeiner, P. Tetrahedron:Asymmetry 2003, 14, 3141–3152.
[3]Takashima S , Nakamura A , Masaki H , et al. Purification and Characterization of Cellulases from Humicola grisea[J]. Journal of the Agricultural Chemical Society of Japan, 1996, 60(1):77-82.
You may like
Application research of Hexapeptide 9
Sep 4, 2025
The study of Fmoc-L-Arg(Pbf)-OH
Aug 15, 2025