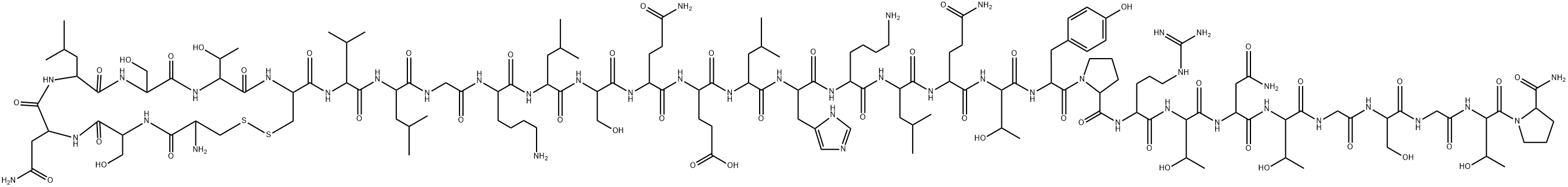
What is Calcitonin salmon?
Description
Osteoporosis is a disease that causes bones to weaken and break more easily. Calcitonin salmon, also known as Salcatonin, is used to treat osteoporosis in women who are at least 5 years past menopause and cannot or do not want to take estrogen products[1]. It is approved by the FDA for the treatment of osteoporosis in women, but not approved for prevention.
Salmon calcitonin and human calcitonin
Calcitonin is a human and synthetic hormone that is also found in salmon. The naturally occurring hormone is involved in calcium regulation and bone metabolism. It works by preventing bone breakdown, increasing bone density (thickness), and decreasing vertebral fracture risk. Salmon calcitonin and human calcitonin inhibit the function of osteoclasts, which are active in the pagetic process; both types of calcitonin preparations come in an injectable form and are approved by the FDA for patients with Paget's disease[2]. Salmon calcitonin therapy is usually initiated at a low dose to ensure patient tolerance and then increased to a daily dose of 100 Medical Research Council units (intramuscularly or subcutaneously). After 6 months of therapy, the patient may receive a maintenance dose of 50 to 100 Medical Research Council units daily.
Side effects
As a polypeptide agent, calcitonin salmon rarely has been reported to cause signs of a systemic allergic reaction including bronchoconstriction, swelling of the throat, hypotension, and tachycardia.
After s.c. injection, the side effects of calcitonin salmon include nausea, vomiting, abdominal pain, dyspepsia, dizziness, flushing, back pain, skin rash, edema of the feet, and rarely polyuria and chills. Typically, adverse effects are related to the specific route of administration of salcatonin. The above adverse effects are less frequent after nasal administration. Nasal administration of salcatonin can cause rhinitis, nasal dryness, epistaxis, sinusitis, and nasal ulcerations.
The European Committee for Medicinal Products (CHMP) for human use found that a higher proportion of patients treated with calcitonin for long periods develop cancer of various types, compared with patients taking a placebo. Consequently, the European Medicines Agency recommended that calcitonin (by injection) be used only on a short-term basis for three conditions for which it had previously been approved in the European Union: Paget's disease, acute bone loss resulting from sudden immobilization, and hypercalcemia caused by cancer[3].
References
[1] Zainabadi K. Drugs targeting SIRT1, a new generation of therapeutics for osteoporosis and other bone related disorders?. Pharmacological Research, 2019; 143: 97-105.
[2] Lane N, et al. Metabolic Bone Disease. Kelley and Firestein's Textbook of Rheumatology, 2017.
[3] Wisneski L, et al. Salmon calcitonin in hypercalcemia. Clinical Pharmacology & Therapeutics, 1978; 24: 219-222.
You may like
Related articles And Qustion
Lastest Price from Calcitonin salmon manufacturers

US $5.00/Box2025-04-28
- CAS:
- 47931-85-1
- Min. Order:
- 1Box
- Purity:
- 99.99%
- Supply Ability:
- 20000 boxes

US $5.00/Box2025-04-21
- CAS:
- 47931-85-1
- Min. Order:
- 1Box
- Purity:
- 99.99%
- Supply Ability:
- 20000 boxes