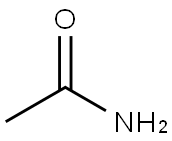
What is Acetamide?
Acetamide is a member of the class of acetamides that results from the formal condensation of acetic acid with ammonia. It is a monocarboxylic acid amide, a N-acylammonia and a member of acetamides. It is a tautomer of an acetimidic acid.
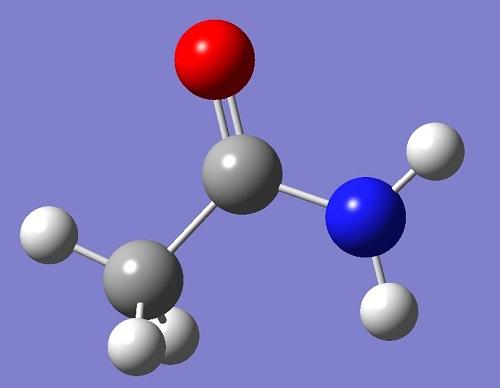
Uses
As a dipolar solvent, acetamide finds many uses as a solvent for both inorganic and organic compounds. The solvency has led to widespread uses in industry including applications in cryoscopy, soldering, and the textile industry. The neutral and amphoteric characteristics allow its use as an antacid in the lacquer, explosives, and cosmetics industries. Its hygroscopic properties make it useful as a plasticizer in coatings, fixtures, cloth, and leather, and as a humectant for paper. It is also a raw material in organic synthesis of methylamine and thioacetamide and as an intermediate in preparation of medicines, insecticides, and plastics.
Mechanism of Toxicity
Acetamide will exist as a vapor in the ambient atmosphere. Atmospheric degradation occurs by reaction with photochemically produced hydroxyl radicals. The half-life for this reaction in air is estimated to be 7.6 days. If released to soil, acetamide is expected to have very high mobility and is not expected to adsorb to suspended solids and sediment. Experiments suggest that this chemical may break down in the environment through biodegradation and not through hydrolysis. Volatilization from water surfaces is not expected to be an important fate process based on this compound’s estimated Henry’s law constant.
Environmental Fate
In primates (including humans), isotretinoin (Accutane) is metabolized to a more active form, 13-cis-4-oxo-retinoic acid, which is able to move through the placental membrane. On its own, however, Accutane (isotretinoin) is not particularly motile across the placental barrier, and perhaps most interestingly tends not to bind to cellular retinoid-binding proteins or nuclear receptors. The rapid isomerization to the all-trans isomer, the oxidation of Accutane (isotretinoin) to 13-cis-4- oxo-retinoic acid, and the relatively high circulation times of these compounds may be important in explaining the teratogenic toxicity of Accutane (isotretinoin).
Some studies have more fully explored the metabolic products of isotretinoin. For example, isotretinoin can be metabolized in the liver by the cytochrome P450 microsomal enzyme system – more specifically the CYP2C8, CYP2C, CYP3A4, and CYP2B6 isoenzymes. The metabolites produced are numerous, including retinoic acid (tretinoin), 4-oxo-isotretinoin, and 4-oxo-retinoic acid (4-oxo-tretinoin). This relatively large array of retinoid metabolites may produce a variety of effects, most notably due to their higher potency as retinoids compared to the parent compound (isotretinoin).
It is possible that these additional metabolites are capable of binding to a variety of retinoid receptors in order to alter gene expression and further transcription or transrepression in protein synthesis, which may be responsible for the toxic effects of isotretinoin.
You may like
Related articles And Qustion
Lastest Price from Acetamide manufacturers

US $0.00/KG2025-04-21
- CAS:
- 60-35-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/KG2025-04-21
- CAS:
- 60-35-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10mt