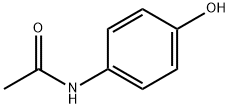
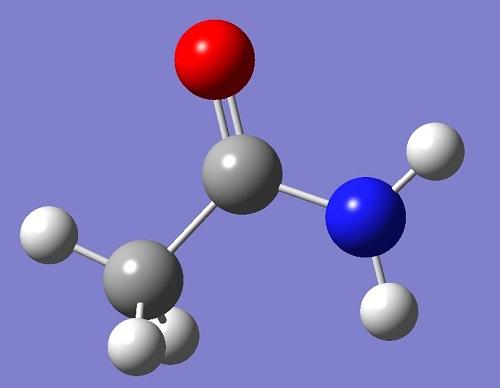
What is Acetaminophen used for?
Acetaminophen can be found as the active ingredient in more than 100 over-the-counter products and a number of prescription drugs, alone or in combination with other drugs. The pharmacology and toxicology of this drug have been extensively studied and reviewed. The first clinical use of acetaminophen dates back to 1893 by von Mering (and subsequently by Hinsberg and Treupel, 1894) as an effective antipyretic with comparable pharmacological effects to antipyrine and phenacetin. However, after a hiatus of almost half a century, acetaminophen was rediscovered as the major metabolite of phenacetin and acetanilide in man and was marketed in the United States as a combination with aspirin and caffeine in 1950.
In the 1960s and 1970s, concerns about gastrointestinal adverse effects of aspirin and methemoglobinemia of acetanilide only led to increased popularity of acetaminophen as a generally safe antipyretic analgesic. Hepatotoxicity of acetaminophen began to be reported in the late 1960s and has been a topic of intense scientific evaluationto this day. The impact of acetaminophen-induced liver toxicity, accidental or otherwise, will be taken up in later sections.

Uses
Acetaminophen is a nonnarcotic analgesic and antipyretic drug. It is used to relieve pain of moderate intensity, such as usually occurs in headache and in many muscle, joint, and peripheral nerve disorders. Headaches are one of the most common indications for the use of acetaminophen. Acetaminophen is used to treat acute tension headaches and mild to moderate migraine, especially in combination with caffeine and aspirin.
Acetaminophen is indicated in chronic pain associated with rheumatoid arthritis, back or hip pain, osteoarthritis, dental pain, or acute pain due to soft-tissue injury. Acetaminophen is a suitable substitute for aspirin for its analgesic or antipyretic uses in cases where aspirin is contraindicated (gastric bleeding) or when the prolongation of bleeding time caused by aspirin would be a disadvantage.
Acetaminophen has been used in studies of pain relief following obstetric and gynecological procedures, including Caesarean section, hysterectomy, tubal ligation, primary dysmenorrhea, and termination of pregnancy. Acetaminophen is also used to manage chronic pain of cancer, postpartum pain, and postoperative pain after minor surgery.
In a double-blind crossover study, the analgesic oral butorphanol, and acetaminophen in combination, showed additive analgesic effects against moderate to severe pain due to metastatic carcinoma over that of the individual drug. Acetaminophen is also widely used as an antipyretic drug to reduce fever.
Environmental Fate
Although a major part of the ingested dose of acetaminophen is detoxified, a very small proportion is metabolized via the cytochrome P450-mixed function oxidase pathway to a highly reactive n-acetyl-p-benzoquinoneimine (NAPQI). The toxic intermediate NAPQI is normally detoxified by endogenous glutathione to cysteine and mercapturic acid conjugates and excreted in the urine. Recent studies have shown that hepatic P450s, CYP2E1, and to a lesser extent CYP1A2 are responsible for conversion of acetaminophen to NAPQI. In acetaminophen overdose, the amount of NAPQI increases and depletes endogenous glutathione stores. Time course studies have shown that covalent binding of reactive NAPQI and subsequent toxicity occur only after cellular glutathione stores are reduced by 70% or more of normal. Mitochondrial dysfunction and damage can be seen as early as 15 min after a toxic dose in mice, suggesting that this may be a critical to cellular necrosis. The NAPQI is then thought to covalently bind to critical cellular macromolecules in hepatocytes and cause cell death.
Recent proteomic studies have identified at least 20 known proteins that are covalently modified by the reactive acetaminophen metabolite. The resulting acetaminophen-cysteine (APAP-CYS) protein adducts can be quantified via a highpressure liquid chromatography coupled with electrochemical detection (HPLC-EC). Hepatic necrosis and inflammation develop as a consequence of hepatocellular death, which results in development of clinical and laboratory findings consistent with liver failure. A similar mechanism is postulated for the renal damage that occurs in some patients following acetaminophen toxicity.
Related articles And Qustion
Lastest Price from Acetaminophen manufacturers

US $0.00-0.00/KG2025-12-18
- CAS:
- 103-90-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00-0.00/kg2025-07-11
- CAS:
- 103-90-2
- Min. Order:
- 1kg
- Purity:
- 99%pure
- Supply Ability:
- 20 tons