What is 3-Oxa-8-azabicyclo [3.2.1] octane, hydrochloride?
3-Oxa-8-azabicyclo [3.2.1] octane, hydrochloride (1:1) (3-OXA-8-AZABICYCLO [3.2.1] OCTANE, HYDROCHLORIDE (1:1); 3-oxa-8-azabicyclo [3.2.1] octane hydrochloride; 3-Oxa-8-aza-bicyclo [3.2.1] octane; 3-Oxa-8-aza-bicyclo [3.2.1] octane HCl; 8-oxa-3-aza-bicyclo; 3-Oxa-8-azabicyclo [3.2.1] octane hydrochloride; (1R,5S)-3-oxa-8-azabicyclo [3.2.1] octane hydrochloride; C6H11NO•HCl) belongs to 8-azacyclic [3.2.1] octane derivative that might possess similar medical efficacy in eating disorders, thermoregulation disorders, sleep and sexual dysfunction, and neurodegenerative disorders symptoms [1,2]. The synthesis of 3-oxa-8-azabicyclo [3.2.1] octane, hydrochloride (1:1) is cumbersome and complicated. The existing synthesis method was less and be presented as following:
3-Oxa-8-azabicyclo [3.2.1] octane, hydrochloride (1:1) could be prepared successfully by using 4-amino-2-(trifluoromethyl) benzonitrile as raw material and 6-step reactions [2]. Firstly, 4-amino-2-(trifluoromethyl) benzonitrile in DCM reacted with triethylamine at 15 minutes and then continue to react with acetoxyacetylchloride for 4 hours at room temperature. The reaction intermediate of 2-{[4-cyano-3-(trifluoromethyl) phenyl] amino}-2-oxoethyl acetate was obtained by separated, washed and dried process. The obtained product that react with LiOH and thionyl chloride in turn would be transformed into 4-[5-(chloromethyl)-1H-tetrazol-1-yl]-2- (trifluoromethyl)benzonitrile, which was added into the mixture of triethylamine and 1,1-dimethylethyl 1-piperazinecarboxylate for reacting 120° for 20 min in a microwave reactor to obtain 1,1-dimethylethyl 4-({1-[4-cyano-3-(trifluoromethyl) phenyl]-1H-tetrazol-5-yl}methyl) -1-piperazinecarboxylate. Then, it was added triethylamine, 4-dimethylaminopyridine and p-toluenesulfonyl chloride for further reaction at room temperature and produce 1,1-dimethylethyl 2-(hydroxymethyl)-5-({[(4-methylphenyl) sulfonyl] oxy} methyl)-1-pyrrolidinecarboxylate. Finally, a suspension of sodium hydride (60% dispersion in mineral oil) in N,N-Dimethylformamide (cooled in an ice bath under argon) was added dropwise a solution of the product of the previous step in N,N-Dimethylformamide (10 mL) and stirred for 5 hours, and then get the target product via multistep treating process, including separating, washing, salt-forming, drying and other process. The yield of this synthesis method is low and the product purification process is complicated.
3-Oxa-8-azabicyclo [3.2.1] octane, hydrochloride (1:1) also could be synthesized by using hexanedioic acid as precursor with overall yield of 23% and purity of 100% [3]. This method could be dived into eight-step reaction (Fig.1), concluding chlorination (sulfoxide chloride), bromination (bromine), benzylamine cyclization (benzylamine), hydrolysis (sodium hydroxide), reduction (sodium borohydride), dehydration condensation (concentrated sulfuric acid), debenzylation hydrogenolysis (hydrogen), and salt-forming (hydrochloric acid).In synthesis process, each reaction is easy to operate with high yield. The obtained 3-Oxa-8-azabicyclo [3.2.1] octane, hydrochloride (1:1) as synthetic intermediate could be used to prepare 3-oxa-8-azabicyclo [3.2.1] octane derivatives, pyridopyrimidine or pyrimidopyrimidine compound with potential pharmaceutical activities [4,5].
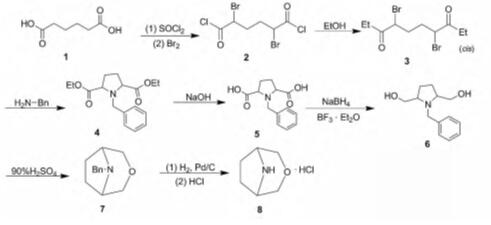
In conclusion, 3-Oxa-8-azabicyclo [3.2.1] octane, hydrochloride (1:1) has been synthesized in complicated and multistep process, which should be simplified by further studying.
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB62465829.htm.
[2] Beswick, P., Gleave, R. J., Hachisu, S., Vile, S., Bertheleme, N., & Ward, S. E. (2015). U.S. Patent No. 9,115,132. Washington, DC: U.S. Patent and Trademark Office.
[3] 王荣宽, 卓广澜, WANGRong-kuan, & ZHUOGuang-Lan. (2016). 3-氧杂-8-氮杂双环[3.2.1]辛烷盐酸盐的合成. 合成化学, 24(11), 998-1001.
[4] https://pubchem.ncbi.nlm.nih.gov/compound/53401129
[5] http://www.chemspider.com/Chemical-Structure.514774.html?rid=8c754cf2-2529-4ed0-9fff-ee8cd21b9328&page_num=
![904316-92-3 3-Oxa-8-azabicyclo [3.2.1] octane, hydrochloride (1:1); Synthesis; Synthetic intermediate](https://www.chemicalbook.com/CAS/GIF/904316-92-3.gif)