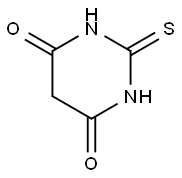
What is 2-Thiobarbituric acid?
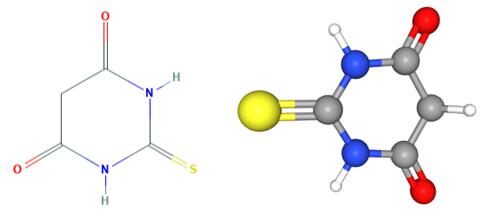
Fig 1. Chemical structure formula and three-dimensional structure of 2-thiobarbituric acid
2-thiobarbituric acid is a barbiturate, the structure of which is that of barbituric acid in which the oxygen at C-2 is replaced by sulfur. It has a role as a reagent and an allergen.
2-thiobarbituric acid is an organic compound and a heterocycle. It is used as a reagent in assaying malondialdehyde (the TBARS assay of lipid peroxidation)[1].It is also used in Kodak Fogging Developer FD-70, part of the Kodak Direct Positive Film Developing Outfit for making black and white slides (positives)[2].
2-Thiobarbituric acid is a molecule made up of heteroatoms: nitrogen, sulphur and oxygen.2-Thiobarbituric acid has been used to measure malondialdehyde (MDA) in lipid peroxidation assay.2-Thiobarbituric acid is useful to quantitate lipopolysaccharides, carrageenan, and sialic acids. It is also used to detect lipid hydroperoxides and lipid oxidation. It has applications in the field of medicine and biochemistry. 2-thiobarbituric acid may possess the ability to prevent metal corrosion.
2-Thiobarbituric acid used to quantitate lipopolysaccharides, carrageenan, and sialic acids; used to detect lipid hydroperoxides and lipid oxidation. 2-Thiobarbituric acid is used in 2-Thiobarbituric acid assay, or 2-Thiobarbituric acid test, which has been employed in the determination of autoxidative alterations of fats and oils.
The wide diffusion of 2-thiobarbituric acid in the scientific literature is due to the 2-Thiobarbituric acid assay, or 2-Thiobarbituric acid test, which has been employed in the determination of autoxidative alterations of fats and oils. Two processes occur in autoxidation, generally: the free radical and the photo-oxidation mechanisms. The better studied is the free radical mechanism. The hydroperoxiepidioxides and bicycloendoperoxides are malonaldehyde precursors. The absorption spectrum obtained with oxidized fatty foods is like the spectrum obtained when 2-Thiobarbituric acid and MDA react. However, during the secondary phase of the autoxidation process other aldehydes (alkanals, 2-alkenals, dienals) are formed which react with 2-Thiobarbituric acid, and they are responsible for off-flavors. Three kinds of pigments (yellow, orange, red adducts) are involved. Also, aromatic aldehydes, which constitute the flavor profile of diverse fruits and essential oils, form with 2-Thiobarbituric acid the characteristic arylidene-2-Thiobarbituric acid acids. Other substances, such as ketones, ketosteroids, acids, esters, sugars, imides and amides, amino acids, oxidized proteins, pyridines, pyrimidines, and vitamins can react with 2-Thiobarbituric acid; they are named TBARS (substances that react with 2-Thiobarbituric acid), and form principally in meats and meat derivatives. Several organic or bio-organic acids, as shikimic and sorbic acids, react photometrically with 2-Thiobarbituric acid if a Malaprade reaction takes place before. A structural study of the red adduct TBA-MDA has been carried out[3].
References
[1] Thiobarbituric acid reactive substances (TBARS) Assay Archived 2006-09-14 at the Wayback Machine, AMDCC Protocols, Animal Models of Diabetic Complications Consortium.
[2] Kodak Direct Positive Film 5246" (PDF). 125px.com. Kodak. Retrieved 6 November 2019.
[3] Guillén-Sans, R, Guzmán-Chozas, M. The Thiobarbituric Acid (TBA) Reaction in Foods: A Review[J]. C R C Critical Reviews in Food Technology, 1998, 38(4):315-350.
[4] https://pubchem.ncbi.nlm.nih.gov/compound/2723628
[5] http://www.chemspider.com/Chemical-Structure.2005830.html?rid=c9cd0427-38a4-484e-a19d-df239dc0bbde
Related articles And Qustion
See also
Lastest Price from 2-Thiobarbituric acid manufacturers

US $0.00/KG2025-04-21
- CAS:
- 504-17-6
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
US $1.10/g2025-04-17
- CAS:
- 504-17-6
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min