What is 2-Acetylaminofluorene?
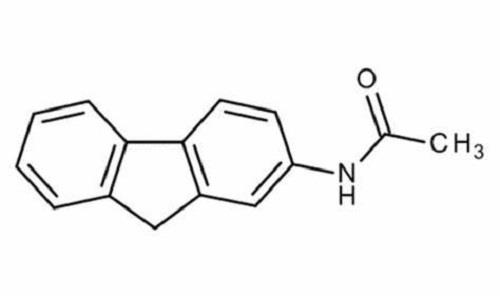
2-Acetylaminofluorene (2-AAF) was originally synthesized to be used as a pesticide but due to its profound carcinogenicity it is now purely used in research laboratories for research purposes only. The occupations at greatest risk to acetylaminofluorene exposure are organic chemists, chemical stockroom workers, and biomedical researchers. 2-AAF is a tan-colored compound insoluble in water (melting point. 194 °C). It is soluble in glycols, alcohols, ether, and acetic acid. 2-AAF is no longer produced in commercial quantities anywhere in the world. In 2009, 2-AAF was distributed in small quantities by 17 specialty chemical companies, including 11 in the United States. As per the US Environmental Protection Agency (EPA), environmental release of 2-AAF rose from w10 000 to w81 000 lb from 1998 to 2001, and then was contained below 1000 lb in 2003. Although neither the National Institute of Occupational Safety and Health (NIOSH) nor the Occupational Safety and Health Administration (OSHA) has estimated the number of US workers exposed to acetylaminofluorene, perhaps fewer than 1000 workers in 200 laboratories may have come in contact with this compound.
Use
2-Acetylaminofluorene is found as a contaminant in coal gasification processes. It was intended to be used as a pesticide but was never marketed due to its carcinogenicity. It has no known use.
Mechanism of Toxicity
According to the US EPA’s Toxics Release Inventory, environmental releases of 2-AAF considerably increased from 1998 to 2001, declined to as low as 255 lb in 2003, and have remained below 1000 lb since 2003. However, most of the releases were to hazardous-waste landfills. In 2007, one facility released about 500 lb of 2-AAF to a hazardous-waste landfill and about 250 lb to air. Release of 2-AAF to the environment from artificial sources is probably not significant since less than 20 lb year of this compound are consumed in the United States. If released to soil, 2-AAF is expected to have low mobility. Chemical hydrolysis, oxidation, and volatilization are not expected to be significant. If released to water, 2-AAF may undergo direct photolysis and is expected to strongly adsorb to suspended solids and sediments. Chemical hydrolysis, oxidation, volatilization, and bioaccumulation are not expected to be significant. If released to the atmosphere, 2-AAF may undergo vapor phase adsorption to airborne particulate matter, it may react with photochemically generated hydroxyl radicals (estimated vapor phase half-life1/4 5.92 h) or it may undergo direct photolysis.
Environmental Fate
4-Acetylaminofluorene is not carcinogenic; 2-AAF is carcinogenic. 2-AAF can be metabolized to form N-hydroxyacetylaminofluorene and 2-aminofluorene, which may covalently bind to the DNA and macromolecules. Ring and N-hydroxylation of 2-AAF leads to the formation of watersoluble conjugates like sulfates and glucuronides. The major fraction of urinary metabolites, around 60–80%, consists of sulfate conjugates, and 10–15% of metabolites in urine consist of glucuronides. 2-AAF has been frequently used to demonstrate stages of multistage carcinogenesis following initiation, driven primarily by carcinogen-induced epigenetic alterations (cytosine DNA methylation, histone methylation, and micro- RNA expression)。