What are the main indications for Regorafenib (Hydrochloride)?
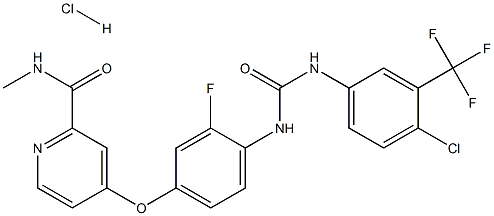
Like fruquintinib, Regorafenib (Hydrochloride) is a small molecule tyrosine kinase inhibitor for the treatment of metastatic colorectal cancer. As an oral multikinase inhibitor, Regorafenib (Hydrochloride) simultaneously blocks angiogenesis (VEGFR 1-3, TIE2), oncogenesis (KIT, RET, RAF1, BRAF), and the tumor microenvironment (platelet-derived growth factor receptor and fibroblast growth factor receptor), thereby inhibiting tumorigenesis, angiogenesis, and the maintenance of tumor microenvironmental signaling.

Regorafenib (Hydrochloride) is primarily indicated for the following:
1. The treatment of patients with metastatic colorectal cancer (mCRC) who have previously received fluorouracil-, oxaliplatin-, and irinotecan-based chemotherapy, or who have previously received or are ineligible for anti-VEGF or anti-EGFR therapy (RAS wild-type).
2. Patients with locally advanced, unresectable or metastatic gastrointestinal stromal tumors (GIST) who have been previously treated with imatinib mesylate and sunitinib malate.
3. Patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
The most common adverse drug reactions (≥30%) in patients treated with Regorafenib (Hydrochloride) are pain, hand-foot skin reaction, weakness/fatigue, diarrhea, decreased appetite and decreased food intake, hypertension and infection.
You may like
Lastest Price from Regorafenib (Hydrochloride) manufacturers

US $0.00/kg2025-03-07
- CAS:
- 835621-07-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons

US $50.00/kg2023-09-07
- CAS:
- 835621-07-3
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 10000MT