Vanadium Nitride: an anode materials
Introduction
Nitrides and oxynitrides are very scarce minerals in natural conditions. Most of them (carlsbergite CrN, osbornite TiN, roaldite (Fe, Ni)4N, nierite α-Si3N4, sinoite Si2N2O) occur solely in extraterrestrial environments, in different types of meteorites. Only siderazot Fe5N2, which is a “grandfathered” and questionable mineral (there is no modern confirmation for composition), appears to be terrestrial in origin and is only identified in fumaroles associations of the Etna and Somma–Vesuvius volcanic complexes. A new mineral, uakitite VN, was observed as an accessory phase in the Uakit iron meteorite and is the sixth nitride mineral found in meteorites. The mineral was approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) as a new mineral species in May 2018 (IMA 2018-003).
Properties
In contrast with the natural phase, synthetic Vanadium Nitride (VN) has been a well-known compound since the 1920s and has been widely used in different branches of industry. Like other transition metal nitrides (CrN, TiN, ZrN, NbN, etc.), it has long been of interest due to its excellent physical and chemical properties, such as high melting point, metallic conductivity, good chemical stability, and high mechanical hardness. Owing to these properties, synthetic VN has a wide range of technological applications, e.g., as an abrasive material, alloying component, wear and corrosion-resistant coatings, field emitter, supercapacitors, superconductors, and buffer layers in microelectronics.
The vanadium–nitrogen phase diagram shows that two stable phases exist: the hexagonal β-V2N and the cubic δ-VN phase. According to the free energy of formation of VN, the nitride can be obtained by heating vanadium metal in a nitrogen atmosphere at 1200°C. However, it has been observed that the reaction rate is appreciable only at around 1350°C.
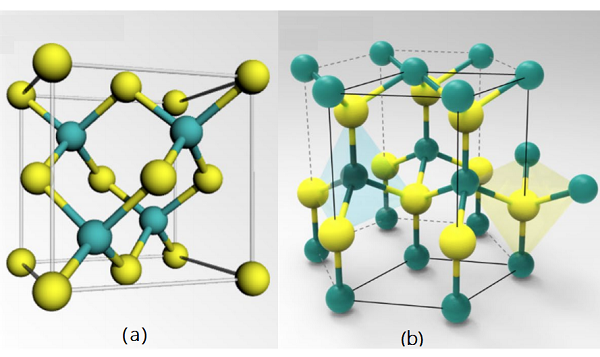
The crystal structure and properties of well-known synthetic VN (or δ-VN) have been studied in detail. It is a cubic NaCl-type structure (Fm-3m, a ≈ 4.135 Å, Z = 4). The δ-VN is isostructural with other transition metal nitrides (CrN, TiN, ZrN, NbN, etc.). The crystal structure of synthetic VN is shown in above[1].
As anode materials
Compared to metal oxides/hydroxides and conducting polymers, transition metal nitrides like Molybdenum Nitride (MoN), Titanium Nitride (TiN), Vanadium Nitride (VN), and Tungsten Nitride (WN) etc. have numerous advantages. For example, transition metal nitrides exhibit considerable voltage range, good sustainability, and high electrical conductivity. Among them, VN has been and continues to be widely investigated by Choi et al. for supercapacitors as anode materials[2].
Among them, VN has been and continues to be widely investigated by Choi et al. for supercapacitors as anode materials. The highest specific capacitance value of 1340 F g−1 at 2 mV s−1 was achieved, and 554 F g−1 was attained at a higher scan rate of 100 mV s−1 between −1.2 and 0 V41. The super high specific capacitance was caused by the high conductivity and nanoscale size of the thin oxide on the surface of the VN nanocrystal. Zhou et al. reported VN was synthesized by calcining V2O5 xerogel, delivering a specific capacitance of 161 F g−1 in 1 M KOH42. The VN nanoparticles ranging from 15 nm to 110 nm achieved a specific capacitance of 186 F g−1 in 1 M KOH at 1 A g−1, as reported by Glushenkov et al. Lu et al. synthesized a porous VN nanowire on a carbon cloth, which showed a specific capacitance of 298.5 F g−1 in 5 M LiCl44. Xu et al. synthesized VN nanofibers via electrostatic spinning and high-temperature calcination in ammonia, which exhibited a high specific capacitance of 291.5 F g−145.
References
[1] Yongtao Tan. “Concise N-doped Carbon Nanosheets/Vanadium Nitride Nanoparticles Materials via Intercalative Polymerization for Supercapacitors.” Scientific Reports (2018): 2915.
[2] I. Galešić, B. Kolbesen. “Formation of vanadium nitride by rapid thermal processing.” Thin Solid Films 349 1 (1999): 14–18.